Metastatic castration-resistant prostate cancer (mCRPC) remains a major therapeutic challenge, particularly after disease progression on androgen receptor pathway inhibitors (ARPIs). While docetaxel has been the standard chemotherapy for mCRPC, its efficacy is often limited, prompting the exploration of novel treatment combinations. Pembrolizumab, an anti–PD-1 immune checkpoint inhibitor, has demonstrated clinical benefit in various malignancies, leading to the hypothesis that its combination with docetaxel might enhance anti-tumor activity in mCRPC. The KEYNOTE-921 trial, a phase III randomized study, was designed to evaluate whether adding pembrolizumab to docetaxel could improve survival outcomes in patients with mCRPC who had previously received ARPIs.
Title: Pembrolizumab Plus Docetaxel Versus Docetaxel for Previously Treated Metastatic Castration-Resistant Prostate Cancer: The Randomized, Double-Blind, Phase III KEYNOTE-921 Trial
Authors: Daniel P. Petrylak, MD, Raffaele Ratta, MD, Nobuaki Matsubara, MD, Ernesto Korbenfeld, MD, Rustem Gafanov, MD, PhD, Loic Mourey, MD, Tilman Todenhöfer, MD, FEBU, Howard Gurney, MBBS, Gero Kramer, MD, Andries M. Bergman, MD, PhD, Pawel Zalewski, MD, Maria De Santis, MD, Andrew J. Armstrong, MD, ScM, FACP, Winald Gerritsen, MD, PhD, Russell Pachynski, MD , Seok Soo Byun, MD, Margitta Retz, MD, PhD, Eric Levesque, MD, PhD, Ray McDermott, MD, PhD , Sergio Bracarda, MD, Ray Manneh, MD, Meital Levartovsky, MD, Xin Tong Li, PhD, Charles Schloss, MD, Christian H. Poehlein, MD and Karim Fizazi, MD, PhD
The KEYNOTE-921 trial, a randomized, double-blind, phase III study, evaluated the efficacy and safety of combining pembrolizumab with docetaxel in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with androgen receptor pathway inhibitors (ARPIs). This article provides a concise overview of the study’s background, methodology, key findings, and conclusions.
Background
Metastatic castration-resistant prostate cancer (mCRPC) remains a significant therapeutic challenge, especially after progression on ARPIs. Docetaxel has been the standard chemotherapy in this setting, but its effectiveness is limited. Immune checkpoint inhibitors like pembrolizumab have shown promise in various cancers, leading to the hypothesis that combining pembrolizumab with docetaxel might enhance treatment outcomes in mCRPC.
Study design and Methods
The KEYNOTE-921 trial was a multicenter, randomized, double-blind, placebo-controlled phase III study conducted across 224 medical centers globally. Eligible participants were assigned in a 1:1 ratio to receive either pembrolizumab plus docetaxel or placebo plus docetaxel. Randomization was stratified based on prior ARPI therapy and sites of metastasis.
Eligible patients had histologically or cytologically confirmed adenocarcinoma of the prostate with disease progression during androgen deprivation therapy or after bilateral orchiectomy. Participants were required to have adequate organ function and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Patients received pembrolizumab (200 mg) or placebo intravenously every three weeks, combined with docetaxel (75 mg/m²) every three weeks and prednisone (5 mg) twice daily. Treatment continued until disease progression, unacceptable toxicity, or a maximum of 35 cycles of pembrolizumab/placebo.
Results
The results of this trial concluded that combining pembrolizumab with docetaxel does not provide a significant survival benefit over docetaxel alone in patients with mCRPC who have progressed on ARPIs. These findings suggest that the current standard of care should remain unchanged, and further research is needed to identify more effective treatment combinations for this patient population.
- Radiographic Progression-Free Survival (rPFS): At the first interim analysis, the median rPFS was 8.6 months for the pembrolizumab plus docetaxel group compared to 8.3 months for the placebo plus docetaxel group (Hazard Ratio [HR], 0.85; 95% Confidence Interval [CI], 0.71 to 1.01; P = .03).
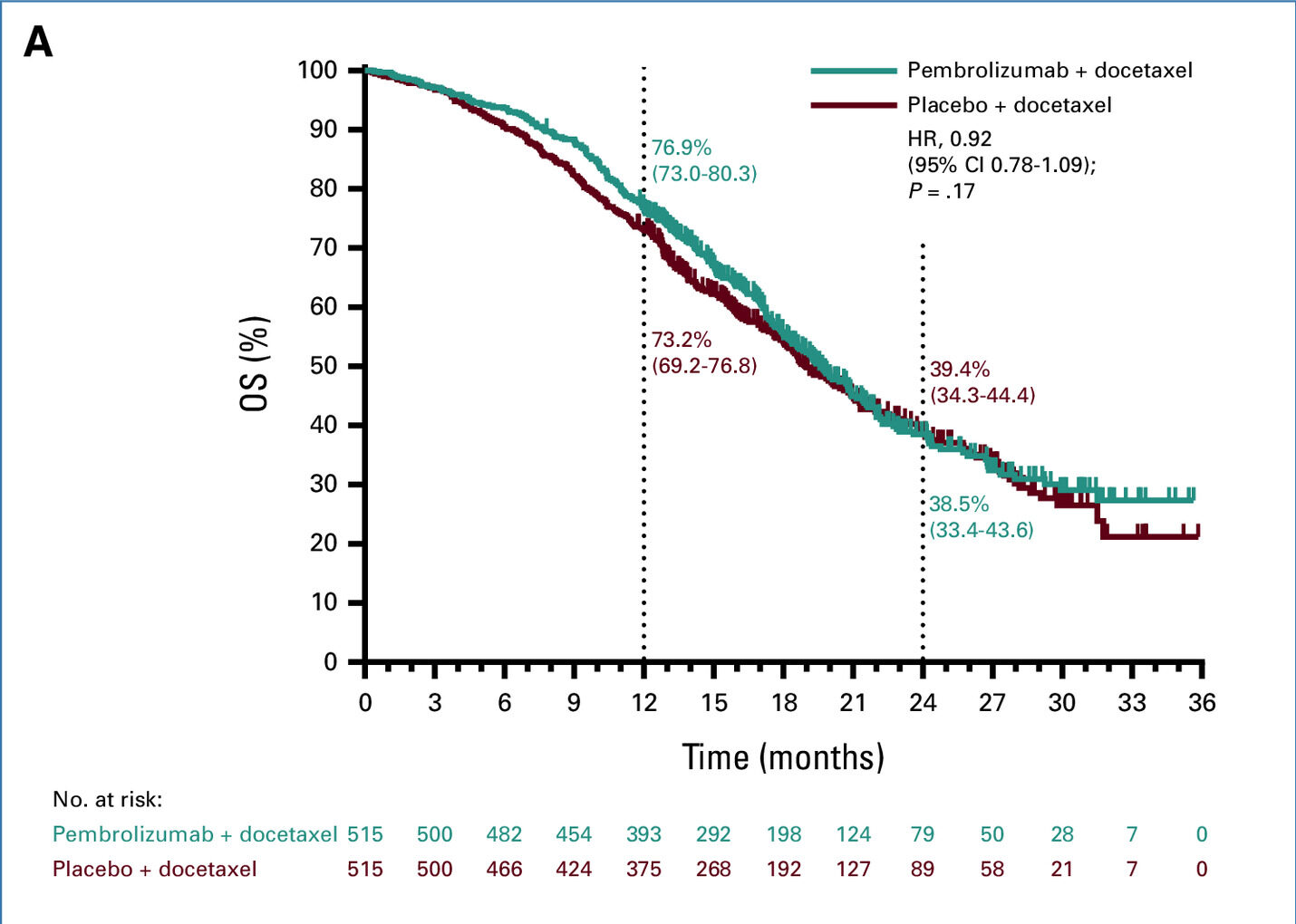
- Overall Survival (OS): At the final analysis, the median OS was 19.6 months in the pembrolizumab plus docetaxel group versus 19.0 months in the placebo plus docetaxel group (HR, 0.92; 95% CI, 0.78 to 1.09; P = .17).
- Safety Profile: Grade ≥3 treatment-related adverse events occurred in 43.2% of patients receiving pembrolizumab plus docetaxel and in 36.6% of those receiving placebo plus docetaxel. Notably, pneumonitis was observed in 7.0% of patients in the pembrolizumab group compared to 3.1% in the placebo group.
Key Takeaway Messages
The addition of pembrolizumab to docetaxel did not result in a statistically significant improvement in rPFS or OS for patients with previously treated mCRPC. While the combination was generally well-tolerated, there was a higher incidence of pneumonitis in the pembrolizumab group.
- No significant survival benefit: Adding pembrolizumab to docetaxel did not significantly improve overall survival (OS) or radiographic progression-free survival (rPFS) in mCRPC.
- Similar median OS: Median OS was 19.6 months (pembrolizumab + docetaxel) vs. 19.0 months (placebo + docetaxel), with no statistical significance (HR, 0.92; P = .17).
- Slight improvement in rPFS: Median rPFS was 8.6 months vs. 8.3 months, but the difference was not clinically meaningful (HR, 0.85; P = .03).
- Higher incidence of pneumonitis: Pembrolizumab-treated patients had a higher rate of pneumonitis (7.0% vs. 3.1%).
- No change in standard of care: Findings do not support pembrolizumab + docetaxel as a new treatment standard for mCRPC after ARPI failure.
- Need for further research: Alternative immune-based strategies should be explored to improve outcomes in mCRPC.
You can read the full article here
Written by Sona Karamyan, MD


