Immunotherapy is a form of cancer treatment that stimulates the body’s immune system to recognize and destroy cancer cells. It has become a cornerstone in the management of various malignancies, offering durable responses and improved survival in cancers like melanoma, lung, and kidney cancer. However, not all patients benefit equally. In some cases, immunotherapy can lead to tumor flare-ups, or the cancer may eventually stop responding—a phenomenon known as acquired resistance. This article explores the complex scenarios where immunotherapy might worsen cancer progression or fail after initial success.
How Does Immunotherapy Work?
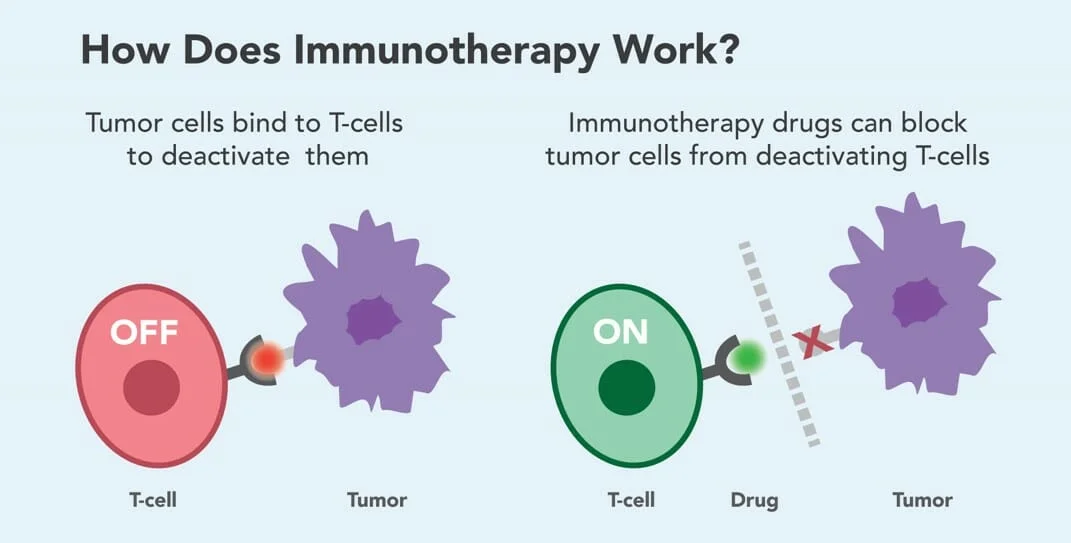
Immunotherapy harnesses and enhances the body’s own immune system to recognize and destroy cancer cells. While the immune system can detect and eliminate abnormal cells, cancer often evades detection by exploiting natural immune checkpoints—proteins that normally prevent the immune system from attacking healthy tissue. Immunotherapy blocks these checkpoints, allowing T cells to remain active against tumors.
A key class of immunotherapy includes immune checkpoint inhibitors, which target molecules like PD-1, PD-L1, and CTLA-4 that cancer cells use to suppress immune responses. Commonly used checkpoint inhibitors include:
-
Pembrolizumab (Keytruda) and Nivolumab (Opdivo) – block PD-1 on T cells
-
Atezolizumab (Tecentriq) and Durvalumab (Imfinzi) – block PD-L1 on tumor cells
-
Ipilimumab (Yervoy) – blocks CTLA-4, boosting T-cell activation early in the immune response
More recently, the development of novel immunotherapy agents such as balstilimab and botensilimab has opened new avenues in cancer treatment, particularly for tumors that have been resistant to conventional immune checkpoint inhibitors. Balstilimab is a next-generation anti–PD-1 monoclonal antibody designed to enhance T-cell activation in a manner similar to pembrolizumab, but with the potential for an improved safety profile and greater flexibility in combination therapies. It works by preventing the PD-1 receptor from binding to its ligands, thereby sustaining the activity of T cells against tumor cells.
Botensilimab, on the other hand, represents a new class of CTLA-4 inhibitors. Unlike traditional CTLA-4 blockers, it has been specifically engineered to selectively modulate different T-cell subsets, which helps reduce toxicity while maximizing immune activation. Botensilimab also stimulates memory T cells and enhances antigen presentation, thereby strengthening the immune system’s ability to recognize and respond to cancer over time.
Can Immunotherapy Make Cancer Worse?
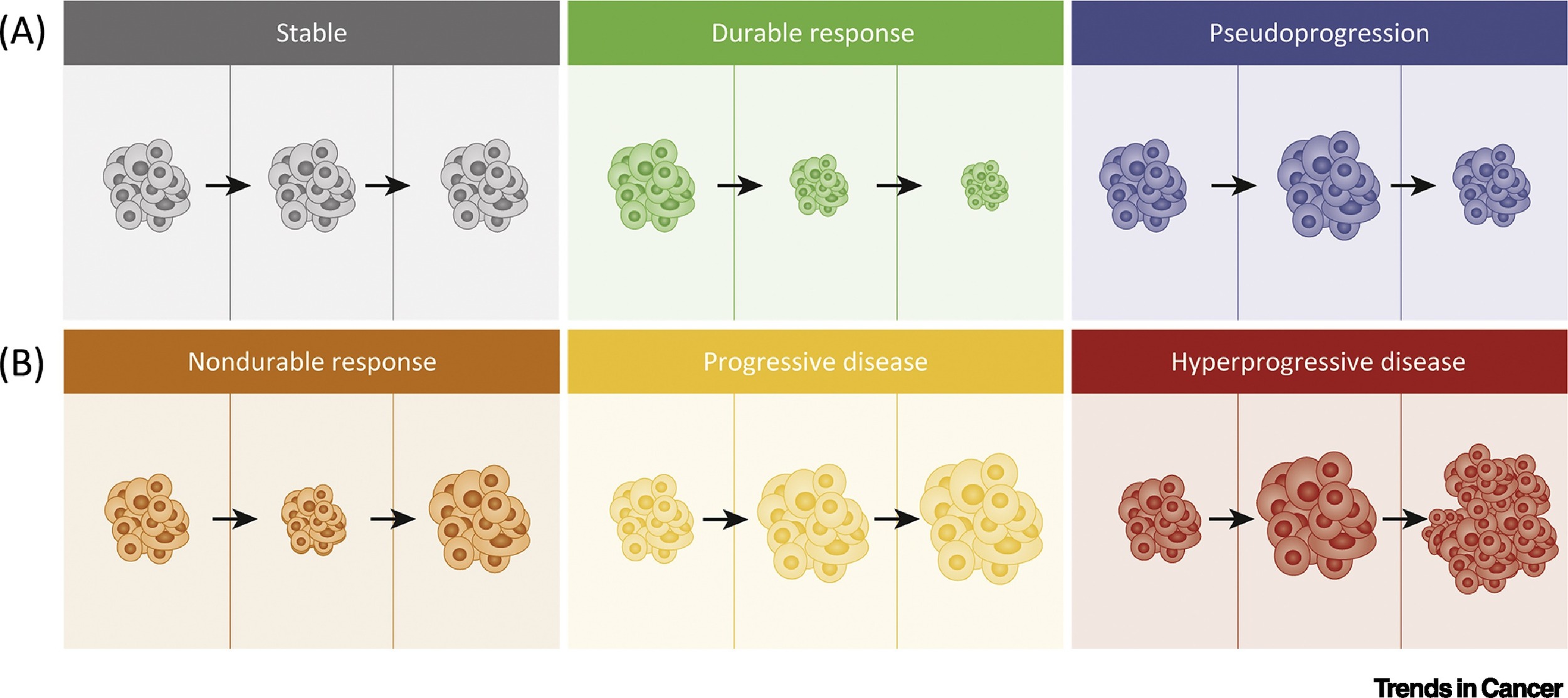
While immunotherapy has transformed cancer care, it does not benefit all patients equally—and in some cases, it may lead to worsening of the disease. A rare but serious phenomenon known as hyperprogressive disease (HPD) can occur, where tumors grow rapidly after starting immunotherapy.
Hyperprogression: When Cancer Grows Faster
Although immunotherapy has transformed cancer treatment, a rare but serious phenomenon known as hyperprogressive disease (HPD) highlights the need for careful patient selection and monitoring. HPD refers to an unexpected and rapid acceleration in tumor growth that occurs shortly after the initiation of immune checkpoint inhibitors. Rather than halting disease progression, immunotherapy in these cases appears to unleash mechanisms that accelerate cancer, resulting in a poor clinical outcome.
The concept of hyperprogression was first systematically described by Champiat et al. in 2017 in Clinical Cancer Research, where HPD was identified in approximately 9% of patients across several cancer types treated with PD-1/PD-L1 inhibitors. Later studies, such as the 2021 JAMA Network Open analysis by Ferrara et al., reported HPD in 16% of patients, with a median overall survival of just 4.6 months, compared to 7.6 months in those without HPD. These findings suggest that for a subset of patients, immunotherapy can paradoxically worsen disease.
The biological mechanisms behind HPD are not yet fully understood, but several hypotheses have emerged. One possibility is that immune checkpoint inhibitors may unintentionally promote tumor-promoting inflammation or disrupt immune regulatory pathways, particularly by altering the behavior of myeloid-derived suppressor cells or regulatory T cells. Another theory points to the role of oncogenic signaling pathways that may become more active under immune pressure. Genetic studies have also helped identify biomarkers that may predict HPD risk. For example, Kato et al. (2017, Cell Death & Differentiation) found that amplifications in MDM2/MDM4 and mutations in EGFR were significantly associated with hyperprogression in patients treated with immunotherapy. Additionally, elevated neutrophil-to-lymphocyte ratios and high levels of LDH have been proposed as clinical indicators of patients who may be more susceptible to this adverse outcome.
In response to the challenges posed by HPD, researchers are working to develop personalized strategies that can mitigate risk. This includes building predictive models that integrate genomic, clinical, and radiological data, and refining the criteria used to define HPD in both trials and clinical practice. For instance, recent efforts focus on identifying early tumor growth dynamics through advanced imaging combined with biomarker analysis. Despite its rarity, hyperprogression underscores the need for vigilant monitoring, especially in patients with high-risk genetic profiles or rapidly growing tumors at baseline. Moving forward, the incorporation of pre-treatment molecular profiling may help guide the selection of patients who are more likely to benefit from immunotherapy—and, just as importantly, identify those for whom it may be harmful.
Why Does Immunotherapy Sometimes Fail?
While immunotherapy has offered new hope in the treatment of many cancers, its success is not universal. A significant number of patients fail to respond, and understanding why is essential to improving outcomes. Several key factors influence the effectiveness of immunotherapy. These include the characteristics of the tumor microenvironment, which may lack sufficient immune cell infiltration or be dominated by immunosuppressive cells; genetic mutations within the tumor that affect antigen presentation or immune recognition; and mechanisms of immune evasion, where cancer cells actively suppress or avoid detection by the immune system. Together, these biological barriers help explain why immunotherapy may be effective for some but not all patients.
Tumor Microenvironment
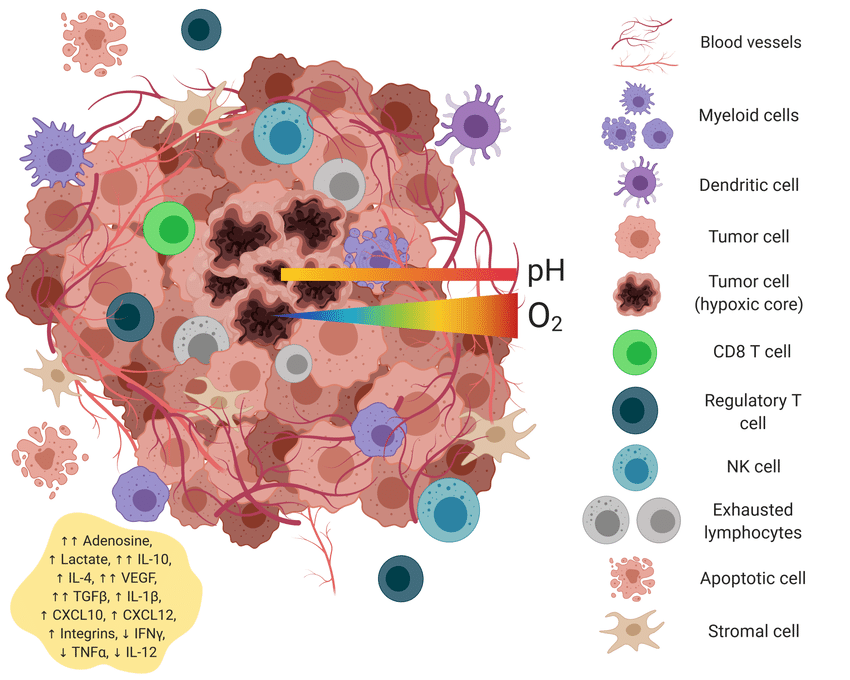
The tumor microenvironment (TME) plays a central role in determining whether a cancer will respond to immunotherapy. Far from being just a mass of cancer cells, the TME is a complex ecosystem made up of immune cells, fibroblasts, blood vessels, signaling molecules, and the extracellular matrix—all of which can influence how the immune system interacts with the tumor. One major reason some tumors resist immunotherapy is that their microenvironment is “immune-excluded” or “immune-deserted.” In these cases, T cells are either absent from the tumor site or unable to penetrate the tumor core, rendering immune checkpoint inhibitors less effective. For example, pancreatic cancer and certain colorectal cancers are known for having dense stromal barriers composed of cancer-associated fibroblasts and extracellular matrix proteins. These structures act as physical and biochemical barriers, preventing immune cells from reaching tumor cells.
Additionally, some tumors promote a highly immunosuppressive environment by recruiting regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). These cells release cytokines such as IL-10 and TGF-β, which inhibit the activity of cytotoxic T lymphocytes and natural killer (NK) cells. This suppressive signaling blunts the immune response, even in the presence of checkpoint inhibitors. Moreover, the low levels of tumor mutational burden (TMB) in some cancers result in fewer neoantigens, making it harder for immune cells to recognize cancer cells as foreign. Without a strong antigenic signal, the immune system remains largely inactive, and immunotherapy has little to amplify.
Genetic Mutations and Resistance
Genetic mutations within cancer cells can significantly contribute to resistance against immunotherapy by altering key pathways involved in immune recognition and response. These mutations enable tumors to evade immune surveillance and diminish the efficacy of treatments such as immune checkpoint inhibitors (ICIs).
- Mutations in Antigen Presentation Pathways: Effective immunotherapy relies on the presentation of tumor antigens to T cells. Mutations in genes like β-2-microglobulin (B2M) can disrupt the formation of major histocompatibility complex (MHC) class I molecules, which are essential for antigen presentation. A study by Zaretsky et al. (2016) reported that loss-of-function mutations in B2M were associated with acquired resistance to the PD-1 inhibitor pembrolizumab in melanoma patients, leading to impaired antigen presentation and reduced T cell recognition of tumor cells.
- Alterations in Interferon Signaling: The interferon-gamma (IFN-γ) pathway is crucial for antitumor immunity. Mutations in Janus kinase 1 (JAK1) and Janus kinase 2 (JAK2) can result in insensitivity to IFN-γ signaling. Zaretsky et al. (2016) also identified truncating mutations in JAK1 and JAK2 in patients with melanoma who developed resistance to pembrolizumab. These mutations led to a lack of response to IFN-γ, thereby reducing the expression of MHC molecules and diminishing the antitumor immune response.
- Activation of the Wnt/β-Catenin Pathway: Aberrant activation of the Wnt/β-catenin signaling pathway has been implicated in immune evasion. Spranger et al. (2015) demonstrated that tumors with active β-catenin signaling exhibited reduced infiltration by dendritic cells and T cells, leading to a non-inflamed tumor microenvironment resistant to ICIs. This suggests that mutations activating β-catenin can create an immunosuppressive milieu that hinders immunotherapy efficacy.
- Loss of PTEN Function: The tumor suppressor gene PTEN negatively regulates the PI3K-AKT signaling pathway. Loss of PTEN function has been associated with resistance to immunotherapy. Peng et al. (2016) found that PTEN loss in melanoma led to decreased T cell infiltration and reduced sensitivity to PD-1 blockade, suggesting that PTEN mutations contribute to an immunosuppressive tumor environment.
- Mutations in DNA Damage Repair Genes: Deficiencies in DNA damage repair genes, such as BRCA1/2, can influence the tumor mutational burden (TMB). While high TMB is generally associated with better responses to ICIs, certain mutations may lead to genomic instability that promotes resistance mechanisms. For instance, alterations in BRCA1/2 have been linked to resistance to various therapies, including immunotherapy, by affecting the tumor’s ability to present neoantigens effectively.
When Immunotherapy Stops Working: What Next?
When immunotherapy stops working, it can be a difficult turning point in a patient’s cancer journey. However, it also marks the beginning of a new decision-making process. Patients and doctors must work together to assess the situation and plan next steps. This may involve confirming true disease progression, evaluating alternative treatments, considering clinical trials, or shifting to supportive care. Early discussions and careful assessment can help guide the best course of action tailored to the patient’s condition and goals.
Alternative Treatments
When immunotherapy ceases to be effective, patients and healthcare providers can consider several alternative treatment options, including chemotherapy, targeted therapy, and radiation therapy. The choice among these depends on factors such as cancer type, disease progression, and the patient’s overall health.
- Chemotherapy: Chemotherapy employs cytotoxic drugs to eliminate rapidly dividing cancer cells. In cases where immunotherapy is no longer effective, chemotherapy can serve as a subsequent treatment. For instance, a study by Schvartsman et al. in Cancer Immunology Research (2017) reported that patients with non-small cell lung cancer who received chemotherapy after progressing on immunotherapy experienced a response rate of approximately 20%, suggesting that prior immunotherapy might sensitize tumors to subsequent chemotherapy.
- Targeted Therapy: Targeted therapies focus on specific molecular abnormalities driving cancer growth. For example, in non-small cell lung cancer patients with epidermal growth factor receptor (EGFR) mutations, tyrosine kinase inhibitors like osimertinib have demonstrated efficacy. A study by Mok et al. in New England Journal of Medicine (2017) showed that osimertinib significantly improved progression-free survival compared to standard chemotherapy in treatment-naïve patients with EGFR-mutated advanced non-small cell lung cancer.
- Radiation Therapy: Radiation therapy uses high-energy particles or waves to destroy or damage cancer cells. It can be particularly effective for localized tumors or as palliative treatment to relieve symptoms. For instance, a study by Theelen et al. in JAMA Oncology (2019) found that adding radiation therapy to immunotherapy in patients with non-small cell lung cancer who had progressed on prior immunotherapy resulted in a median overall survival of 18.4 months compared to 7.5 months with immunotherapy alone.
Combination Therapies
Combining immunotherapy with other cancer treatments—such as chemotherapy, targeted therapy, and radiation therapy—has shown promise in enhancing therapeutic effectiveness. This approach leverages the distinct mechanisms of each modality to improve patient outcomes.
- Chemotherapy and Immunotherapy: Integrating chemotherapy with immunotherapy can enhance the immune system’s ability to recognize and attack tumor cells. Chemotherapy can induce immunogenic cell death, releasing tumor antigens that prime the immune response. A systematic review and meta-analysis by Chen et al. in Frontiers in Pharmacology (2022) evaluated the efficacy and safety of chemotherapy combined with immunotherapy as a first-line treatment for advanced or metastatic squamous non-small cell lung cancer (NSCLC). The study included seven clinical trials with a total of 3,029 patients. Results indicated that the combination therapy significantly prolonged overall survival (OS) and progression-free survival (PFS) compared to chemotherapy alone. However, the combined treatment also resulted in a higher incidence of adverse reactions, underscoring the need for careful patient selection and management.
- Targeted Therapy and Immunotherapy: Combining targeted therapies with immunotherapy aims to disrupt specific oncogenic pathways while simultaneously enhancing the immune response. In metastatic melanoma, the combination of BRAF inhibitors (targeted therapy) with immune checkpoint inhibitors has been explored. A review by Yu et al. in Frontiers in Immunology (2019) discussed the theoretical rationale and clinical practice of this combination. The authors noted that while preclinical models demonstrated significant antitumor activity, clinical trials have faced challenges, including increased toxicity.Nevertheless, the potential for improved outcomes justifies ongoing research into optimizing such combinatorial strategies.
- Radiation Therapy and Immunotherapy: Radiation therapy can modulate the tumor microenvironment, potentially enhancing the efficacy of immunotherapies. A review by Ngwa et al. in Nature Reviews Cancer (2018) outlined five mechanisms through which radiation and immunotherapy may interact to improve clinical outcomes: spatial cooperation, temporal modulation, biological cooperation, cytotoxic enhancement, and normal tissue protection. The authors emphasized the need for further clinical studies to elucidate optimal sequencing and dosing strategies for this combination.
Managing Side Effects and Monitoring Progress
Immunotherapy can trigger side effects known as immune-related adverse events (irAEs), which occur when the immune system attacks healthy tissues. While many are mild, some can be serious or even life-threatening if not detected early. Managing these side effects involves prompt recognition, appropriate use of immunosuppressive medicationslike corticosteroids, and close monitoring through regular check-ups and lab tests. This article highlights the importance of timely intervention and ongoing surveillance to ensure safe and effective treatment.
Common Side Effects
While immunotherapy has brought lasting benefits to many cancer patients, it can also lead to a range of immune-related side effects, as the immune system may begin to attack healthy tissues along with cancer cells. These side effects vary in severity and timing, but most are manageable with early recognition and supportive care.
One of the most common side effects is fatigue, which can be persistent and debilitating. Unlike typical tiredness, immunotherapy-related fatigue often lingers despite rest. To manage it, patients are encouraged to maintain a consistent sleep schedule, stay lightly active (such as taking short walks), eat balanced meals, and allow for regular periods of rest throughout the day. It’s also important to rule out underlying issues such as anemia or thyroid dysfunction, which can contribute to fatigue and are sometimes caused by immunotherapy. Skin reactions are also frequently observed and may include rash, itching, dryness, or, in some cases, more serious inflammatory skin conditions. These usually appear early in treatment and are often mild. Simple interventions like fragrance-free moisturizers, cool compresses, and antihistamines can provide relief. For more severe cases, topical corticosteroids or even systemic steroids may be needed, and a dermatologist may be consulted if symptoms persist or worsen.
Gastrointestinal symptoms, particularly diarrhea and abdominal discomfort, can signal inflammation of the colon (colitis). Mild symptoms can often be managed with dietary changes, hydration, and anti-diarrheal medications. However, persistent or severe diarrhea warrants immediate medical evaluation, as it may require corticosteroids or immunosuppressive therapy to prevent serious complications like bowel perforation or dehydration. Other commonly reported issues include joint pain, dry eyes or mouth, low-grade fever, and endocrine disturbances, such as hypothyroidism or adrenal insufficiency. These require regular monitoring through blood work and clinical evaluation. For endocrine side effects, hormone replacement therapy is typically effective and allows patients to continue immunotherapy safely.
In all cases, communication with the healthcare team is essential. Patients should report new or worsening symptoms promptly. Early intervention often prevents more serious complications and allows for continued treatment without interruption. Ultimately, managing immunotherapy side effects involves a combination of patient education, vigilant monitoring, and supportive care, helping patients remain on treatment while maintaining their quality of life.

Regular Monitoring and Follow-Up
Regular monitoring is a vital part of cancer care during and after immunotherapy. While immunotherapy can offer powerful and lasting responses, its effects can be unpredictable, and careful follow-up is essential to track progress, detect complications, and adjust treatment plans in a timely manner.
Imaging tests, such as CT scans, MRIs, or PET scans, are routinely used to assess how well the cancer is responding to treatment. These scans help doctors determine whether tumors are shrinking, stable, or growing. In some cases, what appears to be tumor growth early in treatment may actually be pseudo-progression, a temporary swelling caused by immune cell infiltration. Regular imaging helps distinguish between real progression and delayed but meaningful responses, guiding whether to continue, pause, or change therapy. Blood tests are equally important. They are used to monitor for organ function, hormonal imbalances, and immune-related side effects. For example, abnormal liver enzymes may indicate immune-mediated hepatitis, and changes in thyroid hormones can signal endocrine side effects like hypothyroidism or adrenal insufficiency. Monitoring inflammatory markers and blood counts can also offer early warning signs of emerging toxicity or immune overactivation. In addition, physical examinations and routine symptom assessments during clinic visits allow healthcare providers to evaluate general health, track side effects, and detect subtle changes that might not appear on tests. These conversations give patients an opportunity to raise concerns about new symptoms, fatigue, pain, or emotional well-being—each of which may signal a need to adjust treatment or add supportive care measures.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
Can immunotherapy worsen cancer?
In rare cases, yes. A phenomenon called hyperprogressive disease (HPD) may cause tumors to grow faster after starting immunotherapy.
What is hyperprogressive disease (HPD)?
HPD is a rapid acceleration of tumor growth seen shortly after initiating immune checkpoint inhibitors. It affects a small percentage of patients.
Why does immunotherapy sometimes fail?
Failure may occur due to factors like immune-suppressive tumor microenvironments, genetic mutations, or lack of T-cell infiltration in the tumor.
What role does the tumor microenvironment play in immunotherapy?
The tumor microenvironment can either support or block immune activity. If it suppresses immune cell function, immunotherapy is less effective.
Are there genetic mutations that cause immunotherapy resistance?
Yes. Mutations in B2M, JAK1/JAK2, PTEN, or activation of Wnt/β-catenin pathways can interfere with immune recognition and response.
What are alternatives if immunotherapy stops working?
Options include chemotherapy, targeted therapy, radiation, or clinical trials. Doctors may also consider combining these approaches with immunotherapy.
How can patients be monitored during and after immunotherapy?
Regular imaging, blood tests, and symptom assessments help detect disease progression or late-onset side effects.
What are common side effects of immunotherapy?
Fatigue, skin rashes, diarrhea, joint pain, and endocrine issues like hypothyroidism are among the most frequent immune-related adverse events.
Can immunotherapy be restarted after progression?
Sometimes. Depending on the type and cause of progression, oncologists may consider re-challenging or combining it with other therapies.
How is hyperprogressive disease diagnosed?
Diagnosis is based on imaging that shows rapid tumor growth shortly after starting immunotherapy, often supported by clinical and lab findings.