Presented at GCOS 2025 by Chris Plummer during the session “Morning with the Masters – How Do I Manage Common Cardiovascular Toxicities in Cancer Patients.” QT prolongation sits at a difficult intersection of oncology and cardiology. Clinicians understand the basics, yet the deeper they look the more uncertainty appears—about how best to measure QTc, which correction to trust, how to interpret “normal,” and when to pause or continue anticancer therapy. A pragmatic approach can reduce avoidable harm while preserving oncologic intent. This summary distils the core ideas: what QT represents, why it matters clinically, how to measure and interpret it reliably, how to act when it is prolonged or torsades de pointes (TdP) occurs, and where future opportunity lies.
What the QT Interval Represents
On the surface ECG, QRS reflects ventricular depolarisation and the T wave reflects repolarisation. The QT interval therefore spans the full cycle of ventricular activation and recovery. Prolonged repolarisation creates electrical heterogeneity across the myocardium—particularly between endocardium and epicardium—forming a substrate for polymorphic ventricular tachycardia. TdP, named for its twisting morphology, is the archetypal arrhythmia associated with excessive QT prolongation. The clinical relevance is immediate: misclassification of QTc can lead to falsely reassuring decisions, treatment missteps, and, in rare cases, sudden death.
Why QT Prolongation Matters to Patients
A single case illustrates the stakes. An early-phase trial participant attended a routine visit with an automated QT of 474 ms (graded as mild). He was discharged, but manual reassessment later showed QTc exceeding 540 ms. He subsequently died suddenly, likely from TdP. The lesson is not to catastrophise every prolonged QT but to respect measurement uncertainty, confirm values, and escalate appropriately. False negatives from machine over-reads or poor manual habits are costly.

Measuring QTc Accurately
Consistency begins with basic discipline. ECG devices estimate heart rate reliably; use the machine’s heart rate rather than calculating RR intervals by hand. For QT itself, manual measurement remains the gold standard. A clear 15-cm ruler across the ECG allows determination of the QT in each readable lead; the value of interest is the longest measurable QT, not an average. Digital images sent by email or messaging apps often distort scale on printing; measuring within presentation software (e.g., drawing a reference rectangle in PowerPoint) avoids printer scaling errors and promotes reproducibility.
A practical visual screen helps: imagine a vertical halfway point between consecutive R waves. If the T wave clearly persists beyond that point, the QT is very likely prolonged. This heuristic lacks sensitivity—especially at slower heart rates—but it is specific enough to trigger a proper manual check.
Handling U Waves and Ambiguous T-Wave Offsets
When prominent U waves blur the end of the T wave, measurement errors multiply. If possible, avoid leads with U-wave interference. If unavoidable, the best-supported technique is the tangent method: draw a tangent along the terminal downslope of the T wave and define QT where that tangent intersects the isoelectric baseline. Uniform application of this method improves inter-observer agreement.
Correcting for Heart Rate: Fridericia over Bazett
Heart rate materially shifts raw QT, necessitating correction. Two near-century-old formulae dominate: Bazett (square root) and Fridericia (cube root). Bazett became ubiquitous largely for historical and computational reasons, but it over-corrects at high rates and under-corrects at low rates. Fridericia’s relationship to heart rate is flatter and more stable, making it the preferred correction in modern practice. A simple spreadsheet can operationalise this: enter the ECG-reported heart rate and the longest QT length (ideally measured in millimetres on the paper scale), and let the sheet compute QTcF consistently across serial ECGs.
Rethinking “Normal”
Published “normal ranges” are at best rough guides. Toxicity grading systems often label 450–480 ms as Grade 1, yet a meaningful fraction of healthy individuals fall outside such bands. Approximately one in ten people could meet a “high-grade” QTc threshold despite being otherwise normal. Consequently, risk conversations must integrate baseline variability, rather than treating rigid numeric cut-offs as absolute truths.
When to Measure—and How Often
A baseline ECG before starting therapy is invaluable. Knowing the patient’s pre-treatment QTc anchors all later decisions. Subsequent checks should occur with meaningful clinical change: dose escalations, the introduction of QT-active co-medications, electrolyte disturbances, symptomatic episodes, or new comorbidities. Many drug-specific monitoring schedules derive from trial protocols rather than robust outcome evidence; clinical judgement should overlay protocol checklists.
Which Drugs Are “High Risk”?
Lists vary because methods differ. Comparative tables, regulatory documents, and pharmacovigilance signals do not always converge. Only a few agents, such as arsenic trioxide, repeatedly land in the highest-risk tier across frameworks. Even then, not all QT prolongation translates to arrhythmia. For example, cohorts exposed to arsenic trioxide show frequent QTc ≥500 ms yet low observed rates of malignant ventricular events when carefully managed. The practical message is to move beyond binary labels and assess real-world risk in context: drug properties, co-medications, patient-specific susceptibility, and monitoring capacity.
A Competing-Risks Frame for Oncology
In metastatic disease, the untreated cancer risk approaches certainty without active therapy, while the absolute risk of lethal TdP—though serious—is typically lower. Decisions about holding or continuing oncologic treatment must therefore live inside a competing-risks frame. The aim is not zero cardiac risk at the cost of oncologic futility, but permissive toxicity: a calibrated level of cardiac risk accepted in order to deliver effective cancer care safely.
Practical Management During Treatment
When alerted to possible QT prolongation, the immediate steps are confirmatory and contextual. Re-measure QTcF manually, compare with any historical ECGs (especially the pre-treatment baseline), and repeat a 12-lead to verify the trend. Parallel to measurement, inventory risk: symptoms (e.g., syncope), all prescribed and non-prescribed drugs, electrolyte status, conduction disease, and organ dysfunction that may amplify exposure to QT-active agents.
Decision thresholds provide a starting scaffold. If QTc remains below roughly 480 ms, continuing therapy with routine vigilance is reasonable. Between 480 and 500 ms, many patients can proceed with treatment while increasing monitoring frequency and correcting modifiable risks. At sustained QTc above 500 ms, the balance usually shifts toward temporarily suspending the QT-prolonging anticancer agent and co-medications, correcting contributing factors, and reassessing before resumption. These thresholds should not be applied mechanically; the patient’s cancer trajectory, alternatives, and preferences matter.
Managing Torsades de Pointes
If malignant arrhythmia occurs or cardiac arrest supervenes, follow advanced life support with defibrillation when indicated. The physiologic priority is to extinguish triggers that exploit the repolarisation substrate. Intravenous magnesium is first-line to suppress early after-depolarisations. Oxygenation should be optimised, and potassium, calcium, and magnesium corrected toward high-normal ranges. Offending QT-active agents should be stopped. Increasing heart rate abbreviates repolarisation and destabilises TdP; short-acting beta-agonists (e.g., isoproterenol) can bridge transiently, though tachyphylaxis limits duration, and temporary pacing is often required when prolonged support is anticipated.
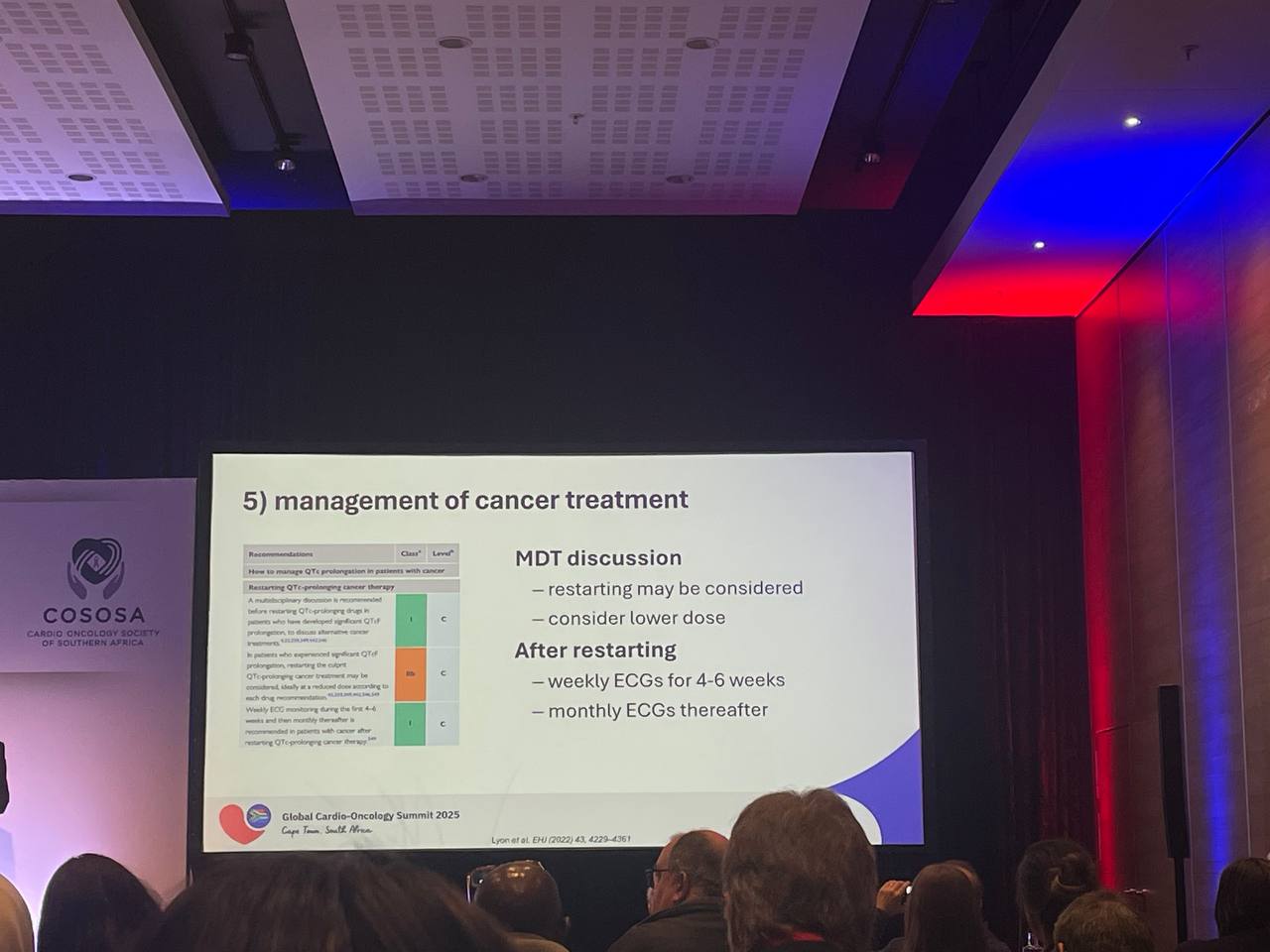
Resuming Anticancer Therapy
Returning to oncologic treatment after significant QT prolongation or TdP is a multidisciplinary decision. Because many effects are dose-dependent, reintroduction at a lower dose is sensible when the anticancer benefit is compelling. A structured surveillance plan improves safety: weekly ECGs for approximately six weeks to capture early dynamics, then monthly checks if stable. Coordination among cardio-oncology, oncology, and pharmacy teams ensures that drug interactions and electrolyte management remain front-of-mind.
The Opportunity for Precision: Data and AI
The field lacks robust, individualised risk tools that integrate drug properties, genetics, comorbidity, electrolytes, and dynamic ECG features to predict TdP in cancer populations. Artificial intelligence offers a route to such precision, but only if fed with large-scale, high-quality, linked datasets and evaluated in prospective workflows. The trajectory is clear: embed decision support within ECG systems and electronic health records to surface a patient-specific absolute risk, not just a flagged number. That future would permit safer permissive toxicity—preserving life-prolonging therapy while minimising arrhythmic events.


