FGD3 is a cellular protein with emerging relevance in breast cancer biology. Although traditionally understudied, recent mechanistic work has identified a dual role for FGD3 in regulating cytoskeletal dynamics, cellular susceptibility to stress, and the immunogenicity of tumor cell death.
This study investigated how FGD3 influences the response of breast cancer cells to anticancer therapies, particularly doxorubicin and the experimental agent ErSO, which induces a unique form of lethal endoplasmic reticulum (ER) stress.
FGD3
Mechanism of Drug-Induced Cell Disruption
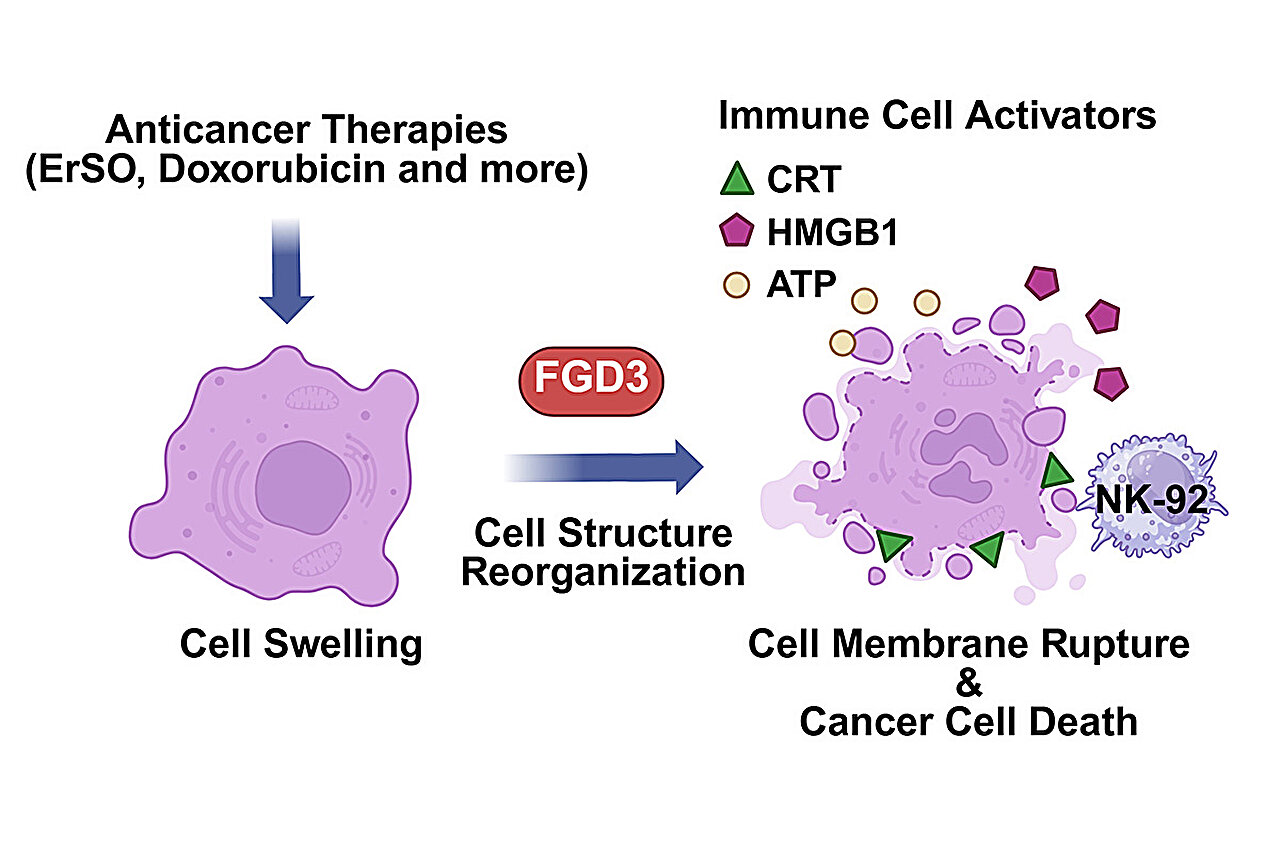
ErSO induces hyperactivation of a stress-response pathway normally used by cancer cells for protection. Unlike typical cytotoxic chemotherapies that inhibit survival pathways, ErSO causes extreme cellular stress, leading to dramatic swelling and eventual rupture of the cancer cell. Doxorubicin can generate a similar state of cellular disruption.
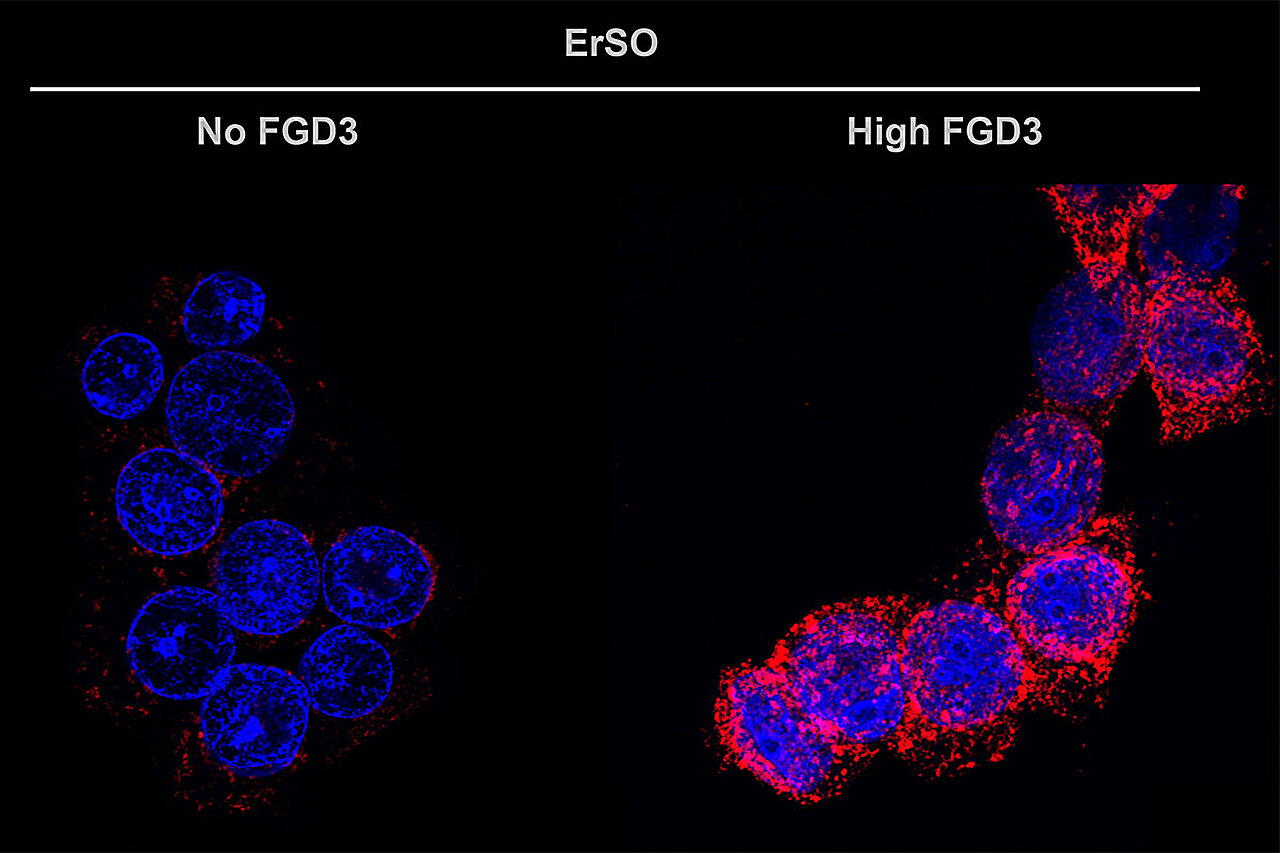
FGD3 was identified as a top regulatory factor in this context. Loss of FGD3 markedly reduced the ability of ErSO to induce cell death, whereas overexpression of FGD3 heightened the disruptive effect, suggesting that FGD3 governs whether stressed cells undergo catastrophic membrane failure.
FGD3’s Dual Role in Cancer Cells
FGD3 exhibits two contrasting functions depending on cellular conditions:
- Baseline state: FGD3 increases cellular flexibility by altering actin cytoskeleton dynamics. This may enhance motility and contribute to metastatic potential.
- Drug-induced stress state: When chemotherapies or ER-stress–inducing agents perturb the cancer cell, high levels of FGD3 destabilize the cytoskeleton, making the swollen cells more prone to rupture. This rupture leads to uncontrolled release of intracellular contents, promoting a form of immunogenic cell death.
FGD3
Immunogenic Consequences of FGD3-Driven Cell Rupture
Rupture of drug-stressed cancer cells exposes danger-associated molecular patterns (DAMPs). A key observation in this study was the robust translocation of calreticulin to the cell surface in FGD3-high cells. Surface calreticulin functions as an “eat me” signal that recruits and activates:
- Natural killer (NK) cells
- Macrophages
Thus, FGD3 enhances the immunotherapy-relevant features of tumor cell death. This mechanism may help overcome the historically limited effectiveness of immunotherapies in solid tumors such as breast cancer.
Experimental Validation in Organoids and Mouse Models
The study utilized several complementary systems to validate the role of FGD3:
- 2D cultured breast cancer cell lines
- 3D patient-derived breast cancer organoids (preserving tumor architecture and protein expression patterns)
- Mouse models of human breast cancer
Across all models, increased FGD3 expression amplified the killing efficiency of both ErSO and doxorubicin. Conversely, reduction of FGD3 impaired drug-induced tumor cell destruction.
Clinical Data Supporting FGD3 as a Predictive Biomarker
Analysis of extensive human breast cancer datasets demonstrated a strong relationship between FGD3 levels and clinical chemotherapy responsiveness. Across multiple chemotherapy classes and breast cancer subtypes:
- High FGD3 expression correlated with favorable treatment response
- Low FGD3 expression correlated with poor responsiveness
These findings suggest FGD3 may serve as a predictive biomarker to identify patients who are more likely to benefit from specific anticancer therapies.
Implications for Cancer Therapy
The dual biology of FGD3 presents meaningful implications:
- Enhancing immunotherapy: FGD3 promotes surface exposure of immune-stimulating molecules, potentially improving response to immunotherapeutic strategies.
- Synergizing with chemotherapy: High FGD3 sensitizes cancer cells to rupture during cytotoxic or stress-inducing treatment.
- Personalized treatment selection: FGD3 expression may guide chemotherapy decisions and dosing strategies.
- Extension to other cancers: Early observations indicate that the FGD3-associated pathway may be relevant across multiple tumor types.
Conclusion
FGD3 represents a mechanistically and clinically significant protein that enhances the effectiveness of both chemotherapy and immunotherapy by promoting immunogenic cancer cell rupture. Its role as a predictive biomarker and therapeutic amplifier opens new opportunities to refine treatment strategies in breast cancer and potentially other malignancies. Further research is warranted to explore therapeutic targeting of FGD3-related pathways and to investigate its applicability across broader cancer contexts.
You Can Read All Article Here


