Etiology, derived from the Greek “aitia” (cause) and “logos” (study), refers to the scientific investigation of the causes or origins of disease. It is a foundational concept in medicine and public health, guiding how clinicians diagnose illness, select appropriate treatments, and design effective prevention strategies. Beyond the clinic, etiological insights are essential for shaping epidemiological models, health policies, and population-level interventions aimed at reducing disease burden.
Etiology, the study of disease causation, is fundamental to oncology—shaping how cancers are diagnosed, treated, and prevented. In cancer research and clinical care, understanding etiology links molecular mechanisms with population-level risk, guiding both precision medicine and public health interventions. Contemporary oncology integrates genetic mutations, environmental exposures, infections, immune dysregulation, metabolic pathways, and psychosocial influences to map the multifactorial origins of cancer. This article explores the evolving science of cancer etiology, from foundational principles to advanced tools in causal inference.
Historical Evolution of Etiological Thought
The quest to understand why diseases arise has transformed dramatically over centuries. In ancient and medieval eras, the predominant explanation for illness was the miasma theory, which held that foul air or “noxious vapors” emanating from decaying matter caused disease. Simultaneously, Hippocratic and Galenic traditions attributed sickness to imbalances of the four humors—blood, phlegm, yellow bile, and black bile—guiding treatments like bloodletting and purgation.
A watershed moment occurred in the 19th century with the advent of the germ theory of disease, championed by Louis Pasteur and formalized by Robert Koch. Koch’s formulation of four postulates provided a rigorous framework for linking specific microorganisms to particular diseases, revolutionizing infectious disease control. These postulates—requiring isolation, culture, and reproduction of disease in healthy hosts—remained the gold standard for pathogen identification well into the 20th century.
As epidemiology matured, researchers recognized that Koch’s criteria were too narrow for many complex diseases. In 1965, Austin Bradford Hill proposed a set of nine guidelines—now known as the Bradford Hill criteria—for inferring causation from statistical associations. These considerations (including strength of association, consistency, biological gradient, and plausibility) enabled scientists to evaluate environmental and behavioral risk factors—such as smoking and lung cancer—where controlled exposure experiments were neither feasible nor ethical.
The late 20th and early 21st centuries ushered in molecular pathology and precision medicine, shifting etiology beyond single agents to multifactorial models. Advances in genomics, proteomics, and epigenomics uncovered how genetic predispositions interact with environmental exposures. For example, research in carcinogenesis revealed that epigenetic modifications can mediate the effects of toxins, while host–microbiome interactions further influence inflammatory pathways in conditions like inflammatory bowel disease.
Today, etiology embraces systems biology frameworks, integrating co-factors—such as diet, microbiota composition, and socioeconomic status—with molecular insights into gene–environment interplay. Causal inference leverages methods from Mendelian randomization to machine learning, allowing investigators to dissect complex, non-linear relationships among diverse factors. This rich, multidimensional approach reflects a fundamental advance: we now appreciate disease causation as a dynamic network, rather than a simple chain of events, guiding more nuanced prevention and personalized treatment strategies.
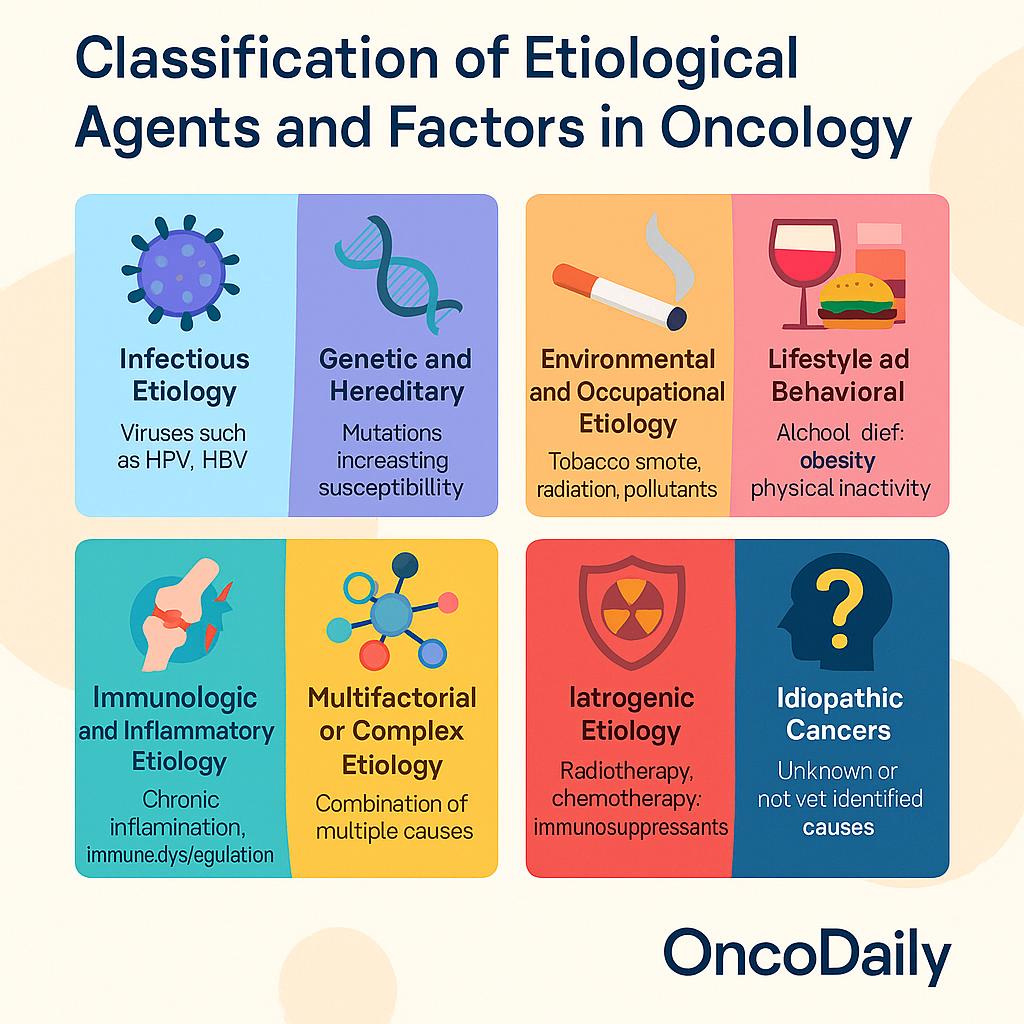
Classification of Etiological Agents and Factors in Oncology
Cancer is a biologically heterogeneous disease with a multifactorial etiology that spans genetic, environmental, infectious, immunologic, and iatrogenic domains. In oncology, understanding the causes of malignancy is crucial not only for prevention but also for risk stratification, early detection, and therapeutic targeting. The following classification provides an overview of the principal etiological categories contributing to carcinogenesis in contemporary cancer medicine.
- Infectious Etiology: A significant proportion of cancers worldwide are attributable to chronic infections, particularly in low- and middle-income countries. Oncogenic viruses such as human papillomavirus (HPV) are directly implicated in cervical, anal, and oropharyngeal cancers through the expression of E6/E7 oncoproteins, which inactivate tumor suppressor genes like p53 and Rb. Hepatitis B virus (HBV) and hepatitis C virus (HCV) contribute to hepatocellular carcinoma by inducing chronic inflammation and hepatic fibrosis. Epstein-Barr virus (EBV) is associated with Burkitt lymphoma, nasopharyngeal carcinoma, and certain gastric carcinomas. Helicobacter pylori, though bacterial, is a well-established cause of gastric adenocarcinoma and MALT lymphoma through mechanisms involving gastritis, epithelial damage, and oncogenic CagA protein delivery. These infectious agents contribute to cancer primarily by inducing chronic inflammation, genomic instability, and immune evasion.
- Genetic and Hereditary Etiology: Germline mutations account for approximately 5–10% of all cancers, although their contribution is far greater in specific subtypes. Hereditary cancer syndromes such as Lynch syndrome (germline mismatch repair mutations), Li-Fraumeni syndrome (TP53 mutations), familial adenomatous polyposis (APC mutations), and BRCA1/2-associated breast and ovarian cancers exemplify direct genetic predisposition to malignancy. These inherited mutations often impair DNA repair pathways or tumor suppressor function, leading to early onset, multifocal disease, and increased lifetime cancer risk. Beyond single-gene syndromes, polygenic risk scores (PRS) are emerging as a tool for estimating cancer susceptibility in the general population, particularly for breast, prostate, and colorectal cancer.
- Environmental and Occupational Etiology: Exposure to carcinogenic agents in the environment and workplace remains a major contributor to cancer incidence. Tobacco smoke—a complex mixture of over 60 known carcinogens—accounts for approximately 85–90% of lung cancers and is implicated in head and neck, bladder, pancreatic, and cervical cancers. Occupational exposures, such as asbestos (mesothelioma, lung cancer), benzene (leukemia), and formaldehyde (nasopharyngeal cancer), have long been recognized by agencies such as IARC as Group 1 carcinogens. Ionizing radiation, whether from medical imaging, radiotherapy, or environmental sources (e.g., radon), is associated with leukemia and solid tumors. Persistent air pollution, particularly PM2.5 and diesel exhaust, is now an established risk factor for lung cancer, even in never-smokers.
- Lifestyle and Behavioral Etiology: Modifiable lifestyle factors are major determinants of cancer risk and are estimated to account for at least one-third of all cancer cases. Dietary patterns rich in processed meats and low in fiber have been associated with colorectal cancer, while obesity is linked to breast, endometrial, esophageal (adenocarcinoma), renal, and pancreatic cancers, likely via hormonal, inflammatory, and insulin resistance pathways. Alcohol consumption is a dose-dependent risk factor for head and neck, liver, esophageal, breast, and colorectal cancers. Physical inactivity contributes indirectly through obesity and metabolic dysregulation. These factors often interact synergistically with genetic and environmental risks to promote tumorigenesis.
- Immunologic and Inflammatory Etiology: Chronic inflammation is a recognized enabling characteristic of cancer. Inflammatory bowel disease, particularly ulcerative colitis and Crohn’s colitis, increases the risk of colorectal cancer through cumulative mucosal damage and epithelial dysplasia. Similarly, chronic hepatitis predisposes to hepatocellular carcinoma, while autoimmune diseasessuch as Sjögren’s syndrome and Hashimoto’s thyroiditis increase the risk of lymphoma and thyroid cancer, respectively. Immunosuppressive states—whether due to HIV, transplant immunosuppression, or congenital syndromes—predispose to virus-associated cancers like Kaposi sarcoma, lymphomas, and HPV-related malignancies. These findings underscore the pivotal role of the immune system in tumor surveillance and the consequences of its dysfunction.
- Multifactorial or Complex Etiology: Most cancers do not arise from a single cause but are the result of complex, multifactorial interactions involving host genetics, environmental exposures, immune response, and stochastic (random) events. For example, lung adenocarcinoma may occur in a never-smoker with EGFR mutation and air pollution exposure, while colorectal cancer may result from low-fiber diet, sedentary behavior, obesity, and low-grade inflammation superimposed on genetic predisposition. The multifactorial model reflects the heterogeneity of tumor initiation pathways, even within the same anatomical site, and has important implications for screening and prevention.
- Iatrogenic Etiology: Medical interventions can also contribute to carcinogenesis. Radiotherapy, while effective in cancer treatment, increases the risk of secondary malignancies in the field of radiation, particularly in pediatric populations and long-term survivors. Certain chemotherapeutic agents—such as alkylating agents and topoisomerase II inhibitors—are associated with therapy-related acute myeloid leukemia (t-AML). Long-term use of immunosuppressants, such as post-transplant agents or biologics in autoimmune disease, can lead to lymphoma or virus-associated cancers. Awareness of iatrogenic risks is critical in the context of survivorship care and therapeutic risk-benefit assessment.
- Idiopathic Cancers: Despite advances in genomics, immunology, and environmental science, a proportion of cancers remain idiopathic, with no identifiable cause. These cases are particularly common in pediatric oncology and among patients without known risk factors or family history. Idiopathic cancers challenge the boundaries of current etiologic frameworks and motivate continued exploration into undiscovered exposures, cryptic genetic variants, and epigenetic dysregulation. As research tools become more refined, some idiopathic cancers may eventually be reclassified under specific causal mechanisms.
Mechanisms of Disease Causation in Oncology
Cancer is fundamentally a disease of dysregulated cellular processes, arising from the interplay between genetic alterations, environmental exposures, and host biological responses. The mechanisms by which these etiological factors initiate and promote oncogenesis are complex and multifactorial, involving cumulative molecular damage, immune evasion, epigenetic remodeling, and tissue microenvironmental shifts. A mechanistic understanding of carcinogenesis is central to modern oncology, guiding biomarker discovery, drug development, and personalized therapy.
- Genetic Mutations and Disrupted Signaling Pathways: The initiating event in many cancers is the accumulation of genetic mutations, either inherited (germline) or acquired (somatic). These mutations may activate oncogenes (e.g., KRAS, MYC, BRAF) or inactivate tumor suppressor genes(e.g., TP53, RB1, PTEN), resulting in deregulated cell proliferation, survival, and genomic instability. Commonly dysregulated signaling cascades in solid and hematologic malignancies include PI3K/AKT/mTOR, MAPK/ERK, and WNT/β-catenin pathways. These disruptions not only drive tumor growth but also influence therapeutic resistance and metastatic potential.
- Microbial Oncogenesis and Immune Evasion: Several infectious agents have direct oncogenic roles. Oncoviruses such as human papillomavirus (HPV), Epstein–Barr virus (EBV), hepatitis B and C viruses (HBV/HCV), and human T-lymphotropic virus type 1 (HTLV-1) can insert viral DNA into host genomes, dysregulate host gene expression, or cause chronic inflammation. For example, HPV-16 and -18 produce E6 and E7 oncoproteins that inactivate p53 and Rb, respectively, in cervical and oropharyngeal cancers. Additionally, cancers frequently develop mechanisms to evade immune surveillance, such as downregulating MHC class I, expressing immune checkpoint molecules (e.g., PD-L1), or recruiting regulatory T cells (Tregs) to the tumor microenvironment. These strategies allow malignant cells to proliferate undetected and are the basis for immune checkpoint blockade therapies in modern oncology.
- Toxin-Mediated Carcinogenesis: Environmental carcinogens, including tobacco smoke (benzo[a]pyrene), asbestos, aflatoxins, and ionizing radiation, induce DNA adducts, chromosomal breaks, and oxidative damage. Such genotoxic insults can initiate mutational events or epigenetic changes that accumulate over time. For example, aflatoxin B1 exposure leads to specific TP53 mutations implicated in hepatocellular carcinoma. Radiation-induced DNA double-strand breaks, if not properly repaired, may drive leukemogenesis or solid tumor formation, particularly in the context of prior cancer therapy (secondary malignancies).
- Oxidative Stress and Epigenetic Dysregulation: Chronic oxidative stress, often arising from inflammation, mitochondrial dysfunction, or exposure to toxins, generates reactive oxygen species (ROS) that damage DNA, proteins, and lipids. Persistent oxidative damage can lead to mutagenesis, chromosomal instability, and tumor-promoting inflammation. In parallel, epigenetic modifications, including hypermethylation of tumor suppressor gene promoters or global hypomethylation, can silence key regulatory genes without altering the DNA sequence. These reversible changes play a central role in cancer initiation and progression and are now being targeted by epigenetic therapies (e.g., DNMT inhibitors in hematologic malignancies).
- Dysbiosis and Microbiome-Driven Tumorigenesis: Emerging evidence links gut microbiota composition to oncogenesis, particularly in colorectal cancer. Dysbiosis—a disruption of the normal microbial balance—may lead to overgrowth of pro-carcinogenic species such as Fusobacterium nucleatum, which can modulate host immunity, promote epithelial invasion, and induce inflammatory signaling. The microbiome also influences drug metabolism, immune responses, and toxicity profiles in cancer patients, making it a growing focus of precision oncology.
- Inflammatory Cascades in Tumor Progression: Chronic inflammation is a well-established hallmark of cancer. Pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β, along with acute phase reactants like C-reactive protein (CRP), create a tumor-permissive environment by promoting angiogenesis, DNA damage, and immune tolerance. Conditions like inflammatory bowel disease, chronic hepatitis, and pancreatitis increase cancer risk via this inflammation-carcinogenesis axis. Additionally, NF-κB and STAT3 signaling play central roles in linking inflammation to proliferation, invasion, and chemoresistance.
- Systems Biology and Network-Level Modeling of Cancer Causation: Oncogenesis is rarely attributable to a single event. Systems biology allows for a holistic view of cancer development by integrating genomic, transcriptomic, proteomic, and metabolomic data into network models. These models can identify master regulators or “hub genes” within disrupted cellular pathways and simulate how perturbations propagate through complex signaling networks. For example, computational modeling of the p53 regulatory network has shed light on how different mutational patterns affect tumor suppression dynamics across tissue types. Such tools are advancing efforts in drug repurposing, target identification, and multi-agent therapeutic design.
Etiological Models in Precision Medicine
The transition from population-based treatment approaches to individualized care lies at the heart of precision medicine, a paradigm deeply rooted in understanding disease etiology. In oncology and other complex diseases, deciphering the causal mechanisms driving pathology enables clinicians to tailor interventions that target the specific biological processes at play—thereby improving therapeutic efficacy while minimizing unnecessary toxicity. Etiological models form the foundation of this approach, allowing medicine to move from “one-size-fits-all” to personalized prevention, diagnosis, and therapy.
One of the clearest demonstrations of this principle is the development of targeted therapies in cancer based on specific molecular drivers. For example, in chronic myeloid leukemia (CML), the identification of the BCR-ABL fusion gene—a constitutively active tyrosine kinase arising from the Philadelphia chromosome translocation—led to the development of imatinib, a targeted inhibitor that transformed a once-fatal disease into a manageable chronic condition. Similar etiologically driven treatments have been developed for EGFR-mutant lung cancer, HER2-amplified breast cancer, and BRAF-mutant melanoma, exemplifying how elucidation of oncogenic pathways directly translates into precision therapeutics.
Beyond somatic mutations, gene–environment interactions are increasingly informing pharmacogenomics—the study of how genetic variation influences drug response. Certain alleles can affect drug metabolism (e.g., TPMT in thiopurine use, UGT1A1 in irinotecan metabolism), while environmental exposures may modulate gene expression or immune activation, influencing both risk and treatment outcomes. Understanding these interactions allows for optimized dosing, improved safety, and avoidance of adverse drug reactions, particularly in oncology where many agents have narrow therapeutic indices.
Biomarker discovery—rooted in etiologic research—has become essential for risk stratification, early detection, and monitoring treatment response. In cancer, biomarkers such as PD-L1 expression, tumor mutational burden, and circulating tumor DNA (ctDNA) guide the selection of immunotherapies, identify minimal residual disease, and forecast recurrence. Etiology-driven biomarker development thus bridges the gap between molecular mechanism and clinical decision-making.
Emerging tools such as polygenic risk scores (PRS) are extending etiological modeling into preventive medicine. By aggregating the effects of numerous common genetic variants, PRS can identify individuals at elevated lifetime risk for cancers like breast, prostate, or colorectal cancer, even in the absence of monogenic syndromes. When integrated with exposomics—the comprehensive analysis of lifetime environmental exposures—and longitudinal patient monitoring(including wearable sensors and digital phenotyping), PRS can inform personalized screening protocols, lifestyle interventions, and surveillance strategies.
Future Directions in Etiological Science
The future of etiological science lies in its evolution from identifying singular disease causes toward modeling complex biological and environmental networks at unprecedented resolution. As the boundaries between disciplines blur, researchers are now equipped with tools that enable high-dimensional analysis of disease origins across time, space, and molecular scale. This shift is transforming our ability to not only understand what causes disease, but how, in whom, and under what circumstances—paving the way for more accurate prediction, earlier detection, and innovative interventions.
A central advancement is the integration of “pan-omic” datasets, encompassing genomics, transcriptomics, epigenomics, metabolomics, proteomics, and exposomics (the comprehensive quantification of environmental exposures over a lifetime). These multilayered data provide a systems-level view of disease causation, allowing researchers to trace dynamic interactions between genes, environmental triggers, metabolic pathways, and immune responses. For example, combining genetic susceptibility profiles with real-time air quality data and dietary patterns can refine risk estimates for lung, colorectal, or breast cancers—offering personalized exposure-risk models that were previously unattainable.
Single-cell and spatial transcriptomics represent another frontier, enabling scientists to map gene expression and cellular behavior within individual tumor microenvironments or inflamed tissues. Unlike bulk sequencing, which averages out cellular heterogeneity, these technologies reveal how rare cell populations, immune infiltrates, and local niches contribute to tumor initiation and progression. Such fine-grained etiological insights may identify new early biomarkers or therapeutic targets, especially in cancer subtypes that resist standard treatment.
The application of deep learning and AI-based models is rapidly accelerating etiological discovery. Trained on vast clinical, molecular, and lifestyle datasets, these models can detect subtle, non-linear patterns that link seemingly disparate variables to disease onset. For instance, AI can integrate wearable sensor data, electronic health records, and polygenic risk scores to predict who might develop aggressive cancers or autoimmune diseases years before symptoms appear. These algorithms may also guide reverse causality assessment and generate new hypotheses for experimental validation.
In parallel, the expansion of longitudinal population biobanks—such as the UK Biobank, All of Us Research Program (U.S.), and FinnGen—is revolutionizing our understanding of disease trajectories. These repositories combine biospecimens, imaging, genomics, and lifelong health records from diverse populations, enabling large-scale studies of disease etiology across age, ancestry, and socioeconomic backgrounds. As data accrues over decades, researchers will be better positioned to model lifetime risk, track preclinical disease phases, and evaluate long-term impacts of early exposures.
Global efforts like the Human Cell Atlas are mapping every human cell type in health and disease, creating a reference framework that supports precise contextualization of etiological events. Combined with planetary health data and cloud-based analytical platforms, these initiatives are poised to unlock new levels of etiologic insight, especially into complex diseases with unclear origins.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What does etiology mean in medicine?
Etiology refers to the study of the causes or origins of diseases. It helps doctors understand how and why a disease develops, which is essential for diagnosis, treatment, and prevention.
Why is understanding cancer etiology important?
Understanding what causes cancer—whether genetic, environmental, or infectious—allows for targeted prevention strategies, early detection, personalized treatments, and better outcomes.
What are the main causes of cancer?
Cancer can be caused by genetic mutations (inherited or acquired), chronic infections (like HPV or H. pylori), environmental exposures (such as tobacco or radiation), inflammation, and lifestyle factors like diet and obesity.
What are hereditary cancer syndromes?
These are inherited genetic conditions that increase cancer risk. Examples include Lynch syndrome, BRCA1/2 mutations, and Li-Fraumeni syndrome. They often involve mutations in tumor suppressor or DNA repair genes.
How do infections cause cancer?
Oncogenic viruses (like HPV, HBV, EBV) and bacteria (like H. pylori) can cause chronic inflammation or insert genes that disrupt normal cell control, leading to cancer over time.
Can lifestyle changes prevent cancer?
Yes, avoiding smoking, maintaining a healthy weight, eating a balanced diet, and limiting alcohol can reduce cancer risk significantly. Screening and vaccination (like HPV) are also effective preventive tools.
What is the role of epigenetics in cancer etiology?
Epigenetic changes—like DNA methylation or histone modification—can turn genes on or off without changing the DNA sequence. These changes may promote cancer development and can sometimes be reversed.
What is the microbiome's role in cancer?
An imbalanced gut microbiome (dysbiosis) may influence inflammation, immunity, and even cancer drug response—especially in colorectal cancer.
How do doctors identify the cause of cancer in a patient?
Doctors use a combination of family history, genetic testing, environmental exposure assessment, and molecular profiling to identify likely causes or contributing factors.
What is iatrogenic cancer?
This refers to cancers caused by medical treatments, such as radiation therapy or chemotherapy. Though rare, they are a known long-term risk in some cancer survivors.


