AstraZeneca and Daiichi Sankyo have announced that the combination of Enhertu (trastuzumab deruxtecan) and pertuzumab has demonstrated a highly statistically significant improvement in progression-free survival (PFS) for patients with previously untreated HER2-positive metastatic breast cancer (MBC). The result is based on data from the DESTINY-Breast09 Phase III trial, which evaluated Enhertu plus pertuzumab versus the standard regimen of trastuzumab, pertuzumab, and a taxane.
Overview of HER2-positive breast cancer
HER2-positive breast cancer is a subtype of breast cancer characterized by overexpression or amplification of the HER2 gene (human epidermal growth factor receptor 2). This gene encodes a protein receptor on the surface of cells that promotes cell growth. When overactive, HER2 drives aggressive tumor growth.
Accounts for approximately 15%–20% of all breast cancer cases and is associated with poor prognosis due to high proliferation rates and early metastatic spread. Nevertheless the outcomes have dramatically improved with the advent of HER2-targeted therapies.
What are targeted therapis for HER2-positive BC?
HER2-targeted therapies have significantly improved outcomes for patients with HER2-positive breast cancer, a subtype defined by overexpression or amplification of the HER2 (human epidermal growth factor receptor 2) gene. These therapies act on distinct targets within or related to the HER2 signaling pathway. Monoclonal antibodies such as trastuzumab and pertuzumab bind to different extracellular domains of the HER2 receptor—trastuzumab to domain IV and pertuzumab to domain II—thereby blocking downstream signaling and preventing dimerization.
Antibody-drug conjugates like trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) deliver cytotoxic agents directly to HER2-expressing cells. Meanwhile, tyrosine kinase inhibitors (TKIs) such as lapatinib, neratinib, and tucatinib target the intracellular tyrosine kinase domain of HER2, inhibiting signaling from within the cell. Together, these agents offer a multifaceted approach to disrupting HER2-driven tumor growth and progression, forming the foundation of modern HER2-positive breast cancer treatment across all stages.
What is the standard treatment of HER2 positive Breast cancer?
First-Line Treatment
The combination of trastuzumab, pertuzumab, and a taxane (THP) remains the standard first-line approach in most patients with HER2-positive MBC.
Second-Line Treatment
The DESTINY-Breast03 trial established trastuzumab deruxtecan (T-DXd) as the preferred second-line option, demonstrating significantly improved progression-free survival (PFS) and a trend toward improved overall survival (OS) compared to trastuzumab emtansine (T-DM1).
What Drug is Trastuzumab Deruxtecan and How Does it Work?
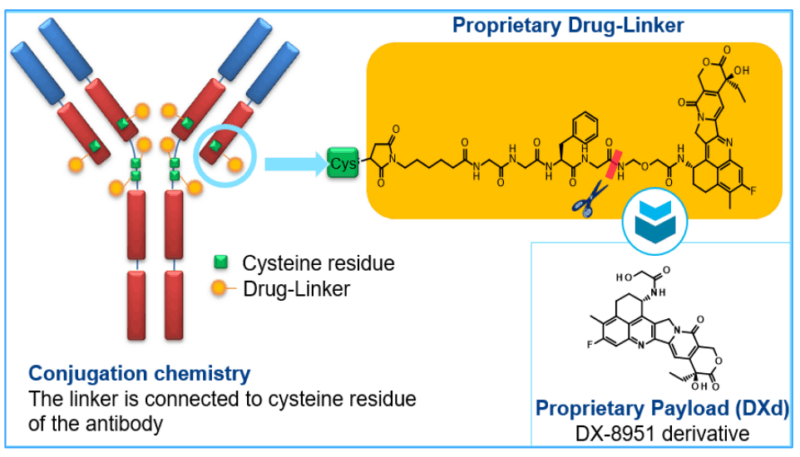
Trastuzumab Deruxtecan (ENHERTU®) is an innovative HER2-directed antibody-drug conjugate (ADC) developed by Daiichi Sankyo in collaboration with AstraZeneca.

Trastuzumab Deruxtecan consists of a HER2-targeted monoclonal antibody (trastuzumab) attached to a cytotoxic chemotherapy agent (deruxtecan, a topoisomerase I inhibitor) via a stable linker. The antibody component of the drug binds specifically to HER2 receptors on tumor cells. Once bound, the drug is internalized by the cancer cell, releasing the potent chemotherapy agent (deruxtecan), which interferes with DNA replication and induces cell death.
This dual mechanism—targeting HER2 for specific binding and delivering a potent chemotherapy agent directly into the cancer cell—is what makes trastuzumab deruxtecan a highly effective ADC. This specificity helps reduce damage to normal cells, minimizing side effects compared to traditional chemotherapy.
The DESTINY clinical trials are a series of pivotal studies evaluating the efficacy and safety of trastuzumab deruxtecan (T-DXd, ENHERTU®) in patients with HER2-positive and HER2-low cancers across different tumor types. These trials have demonstrated significant improvements in progression-free survival (PFS) and overall survival (OS) compared to standard therapies, leading to regulatory approvals worldwide.
Daiichi Sankyo and Astrazeneca recently issued a press release about the latest outcomes of the DESTINY-Breast09 Phase III trial.
DESTINY-Breast09 Trial and what it changed.
DESTINY-Breast09 is a global, multicenter, randomized, open-label Phase III trial designed to assess the efficacy and safety of Enhertu (5.4 mg/kg) either alone or in combination with pertuzumab, compared to the standard first-line regimen of trastuzumab, pertuzumab, and a taxane (THP: docetaxel or paclitaxel) in patients with HER2-positive metastatic breast cancer.
Participants were randomized in a 1:1:1 ratio to receive either Enhertu monotherapy with a placebo for pertuzumab, Enhertu plus pertuzumab, or the standard THP regimen. Randomization was stratified based on prior treatment setting (de novo metastatic vs. progression from early-stage disease), hormone receptor (HR) status, and PIK3CA mutation status.
- Primary Endpoint: Progression-free survival (PFS) by blinded independent central review.
- Secondary Endpoints: Investigator-assessed PFS, overall survival (OS), objective response rate (ORR), duration of response (DoR), time to second progression, PROs, pharmacokinetics, and safety.
A total of 1,157 patients were enrolled across trial sites in Africa, Asia, Europe, North America, and South America.
The trial met its primary endpoint, showing a significant PFS benefit for the Enhertu-based regimen. Secondary endpoints, including overall survival (OS), are still being evaluated. The safety profile of the Enhertu combination was consistent with previous studies, with no new safety concerns identified.
These results suggest that the Enhertu and pertuzumab combination could become a new first-line treatment option for patients with HER2-positive metastatic breast cancer, potentially replacing the current standard of care.


