CRISPR-Edited Islet Transplant results from a landmark first-in-human study show that genome-engineered allogeneic islet cells can be transplanted without any immunosuppression, remain immune-silent, and maintain glucose-responsive insulin secretion in a person with long-standing type 1 diabetes. The findings offer a glimpse into a future where cell replacement therapy could potentially treat type 1 diabetes without the toxic burden of lifelong anti-rejection drugs.
Title: Survival of Transplanted Allogeneic Beta Cells with No Immunosuppression
Authors: Per-Ola Carlsson, M.D., Ph.D., Xiaomeng Hu, Ph.D., Hanne Scholz, Ph.D., Sofie Ingvast, B.Sc., Torbjörn Lundgren, M.D., Ph.D., Tim Scholz, M.D., Ph.D., Olof Eriksson, Ph.D., Per Liss, M.D., Ph.D., Di Yu, Ph.D., Tobias Deuse, M.D., Olle Korsgren, M.D., Ph.D., Sonja Schrepfer, M.D., Ph.D.
Background
For decades, clinical transplantation of allogeneic organs and cells has depended on immunosuppressive therapy. Although agents like cyclosporin A revolutionized transplantation, they introduced major toxicity, infection risk, metabolic complications, cardiovascular events, and long-term mortality.
In type 1 diabetes, exogenous insulin therapy—despite a century of progress—remains a treatment rather than a cure. Many patients continue to face reduced quality of life, elevated cardiovascular risk, and shortened survival.
Preclinical studies have shown that “hypoimmune” gene-edited islet cells, with deleted HLA expression and overexpression of the innate-immune “don’t-eat-me” signal CD47, can evade both alloimmunity and autoimmunity in humanized mouse models and nonhuman primates. The current study brings this approach into the clinic for the first time.
Study Overview
A 42-year-old man with a 37-year history of type 1 diabetes (HbA1c 10.9%, no measurable C-peptide) received allogeneic donor islet cells that had been extensively genetically modified using CRISPR–Cas12b editing and lentiviral CD47 overexpression.
Key edits included:
- B2M knockout → loss of HLA class I
- CIITA knockout → loss of HLA class II
- CD47 overexpression → protection from macrophage and NK-cell killing
The final product (UP421) contained a mix of fully edited hypoimmune islet cells, double-knockout cells with endogenous CD47, and residual wild-type cells.
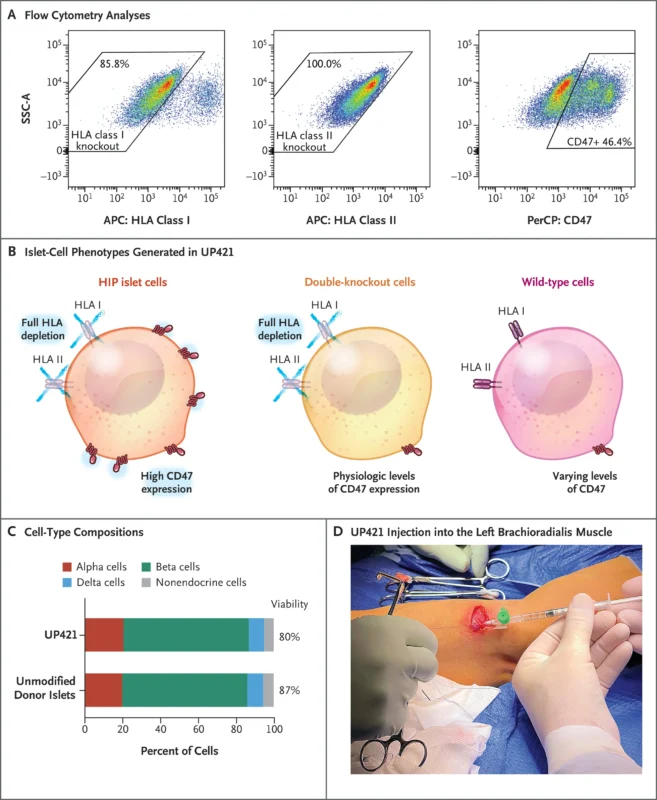
A total of 79.6 million engineered islet cells were implanted into the forearm muscle through 17 microinjections. No glucocorticoids, anti-inflammatory agents, or immunosuppressive drugs were given.
Immune Response
Over 12 weeks, investigators monitored immune reactivity toward each islet-cell subpopulation:
- Wild-type cells triggered strong T-cell and antibody responses and were eliminated.
- Double-knockout cells (HLA class I/II deficient but without high CD47) triggered innate immune killing.
- HIP hypoimmune cells caused no T-cell activation, no NK or macrophage killing,no antibody induction or resistant to complement and cytotoxicity assays.
- Despite robust immune responses to non-edited cells, HIP cells remained fully protected.
Graft Function
C-peptide testing demonstrated:
- Stable basal C-peptide throughout 12 weeks
- Glucose-responsive C-peptide release on mixed-meal tolerance tests at weeks 4, 8, and 12 (no C-peptide at baseline)
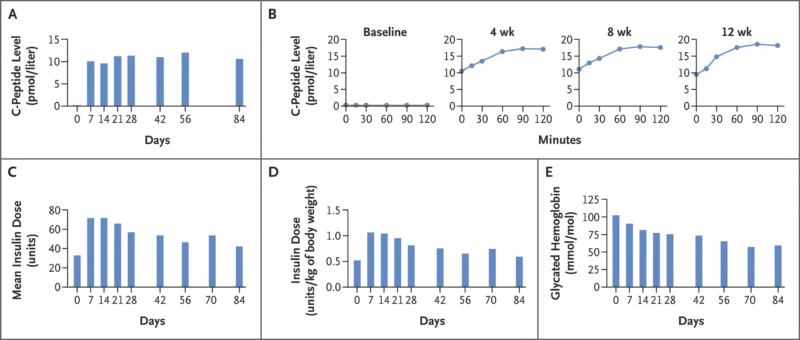
Insulin dosing was intentionally increased early after transplantation to protect the graft from hyperglycemic stress. HbA1c decreased by ~42%, driven mainly by exogenous insulin.
PET-MRI imaging confirmed:
- Clear graft visualization
- GLP-1R tracer uptake, indicating viable, endocrine-active transplanted islets
- No inflammation or pathologic tissue changes
Safety
Four mild adverse events occurred (e.g., thrombophlebitis, local paresthesia), none serious or drug-related. There were:
- No infections
- No immune-related complications
- No systemic inflammatory events
- Interpretation
This early clinical experience provides strong evidence that:
- Hypoimmune gene editing can render allogeneic islets invisible to both adaptive and innate immunity.
- Functional insulin secretion can be achieved without immunosuppressive therapy.
- Forearm muscle may serve as a practical and vascularized implantation site.
Although the study used a very small islet mass (7,1% of a therapeutic dose), the functional C-peptide response suggests that mean (±SD) of 11,547±1604 islet equivalents per kilogram of body weight,11 with 1560 cells per islet equivalent,12 is capable of producing sustained insulin independence.
Broader Implications
The platform could be applicable beyond islet cells—potentially enabling immune-evasive transplantation of cardiomyocytes, neurons, stem-cell–derived tissues, or even engineered organoids.
The study demonstrates a paradigm shift. Rather than inducing immune tolerance, immune evasion through precision gene editing may offer a practical path toward universal, off-the-shelf cell therapies.
You can read the full article here.


