What is Rhabdomyosarcoma?
Rhabdomyosarcoma is a type of soft tissue sarcoma that typically begins in early skeletal muscle cells. It develops in a type of muscle called striated muscle, which are the voluntary muscles of the arms, legs, and rest of the body that people can control. Most cases are diagnosed in children under the age of 6. Although rhabdomyosarcoma can arise anywhere in the body, there are distinct age and type involvement patterns.
Children and young adolescents have more head and neck involvement which is usually of the embryonal type. Extremity rhabdomyosarcoma is more common in adolescents and usually the alveolar type. Botryoid rhabdomyosarcoma occurs in hollow viscera, usually in children. Pleomorphic rhabdomyosarcoma typically arises in the extremities and mainly affects adults. Common rhabdomyosarcoma metastatic sites include lung, bone marrow, and lymph nodes.
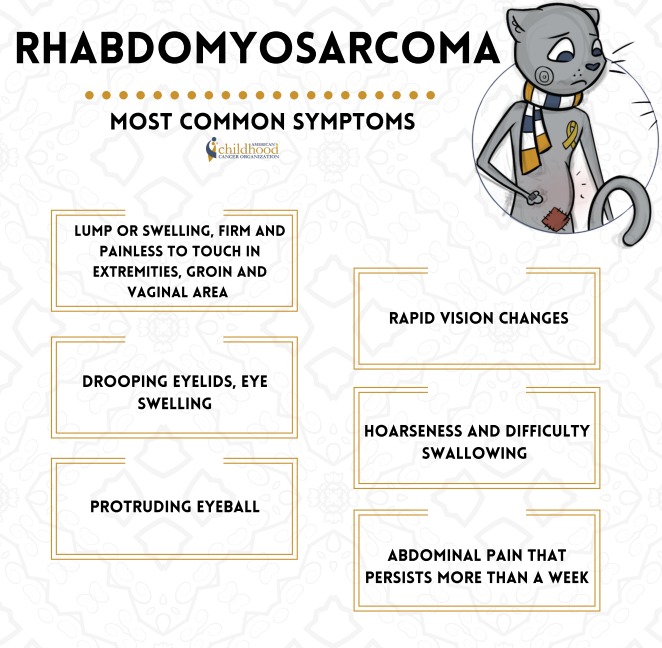
Common symptoms of rhabdomyosarcoma. This image is taken from acco.org.
Types of Rhabdomyosarcoma
There are 2 major subtypes:
- Alveolar rhabdomyosarcoma (ARMS).
- Embryonal rhabdomyosarcoma (ERMS).
Both subtypes pose substantial clinical challenges because achieving a cure requires controlling the primary tumour (which may arise in a wide variety of anatomical sites) by surgical resection and/or ionizing radiation and eradicating systemic metastatic disease using intensive chemotherapy.
The World Health Organization (WHO) also recognizes two rarer RMS subtypes:
- Pleomorphic RMS is a morphological variant of RMS that typically occurs in adults.
- In children, a spindle cell/sclerosing RMS variant is seen. Those tumors arising in the head/neck region seem to be more likely to carry specific somatic mutations and have a poorer prognosis.
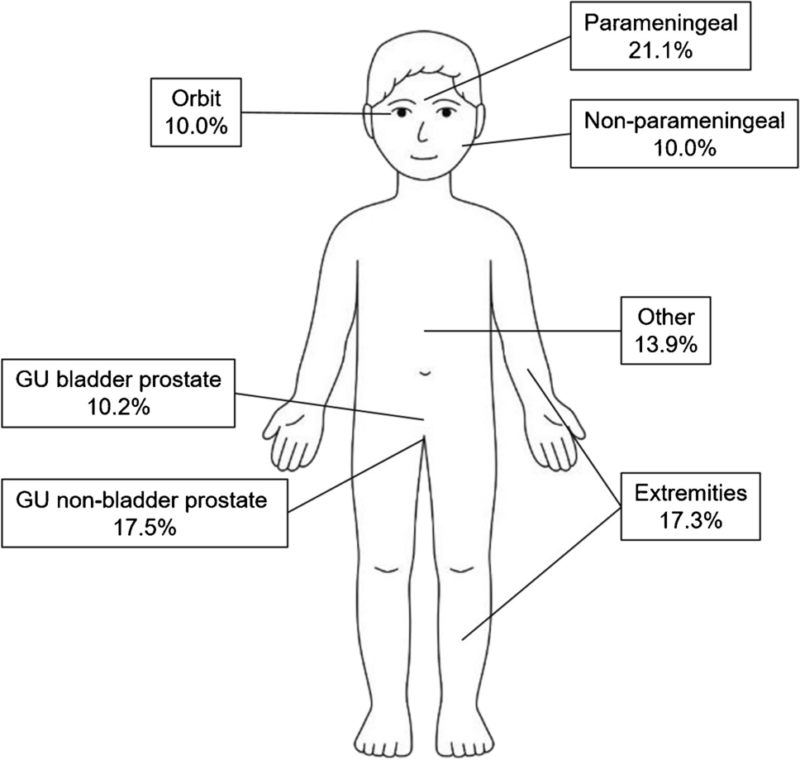
Distribution of rhabdomyosarcoma by primary site, based on data registered in the International Soft Tissue Sarcoma Consortium’s database (n = 6,809 patients). This image is taken from springer.com.
Prevalence and Demographics
Incidence
- Rhabdomyosarcoma accounts for about 3% of all childhood cancers.
- There are approximately 400-500 new cases diagnosed each year in the United States.
- The incidence rate is around 4.71 per million children and adolescents under 20 years old in the US.
Ethnicity and Race
No particular race or ethnic group seems to have an unusually high rate of rhabdomyosarcoma.
Age and gender
- More than half of the cases occur in children younger than 10 years old.
- There is a peak in incidence during early childhood (under 4 years old) at 6.5 per million.
- A second, less pronounced peak occurs during adolescence, possibly related to pubertal growth.
- Rhabdomyosarcoma is slightly more common in males than females, with a male-to-female ratio of 1.37:1.
- This male predominance is driven by the embryonal subtype (male-to-female ratio 1.51:1).
Symptoms of Rhabdomyosarcoma
The symptoms of rhabdomyosarcoma can vary depending on the location and size of the tumor. Some common symptoms include:
- Lumps or swelling in the affected area (e.g., head, neck, arms, legs, or trunk)
- Earache or nosebleeds (if the tumor is located in the head or neck region)
- Vomiting or difficulty swallowing (if the tumor is located in the abdomen or chest)
- Bulging of the eye or vision problems (if the tumor is located near the eye)
- Persistent cough or breathing difficulties (if the tumor is located in the chest)
Other potential symptoms as the cancer advances include bone pain, persistent cough, weakness, and weight loss. Jaundice may occur if the tumor affects the bile ducts. The symptoms are often initially mistaken for a minor injury or infection. However, any persistent lump, swelling, or other worrisome symptom in a child should be evaluated promptly by a doctor.
Causes and Risk Factors
Genetic Factors
Children with certain rare inherited genetic syndromes have a higher risk of developing rhabdomyosarcoma, including:
- Costello syndrome
- Cardio-facio-cutaneous syndrome
- Neurofibromatosis type 1
- Noonan syndrome
- Li-Fraumeni syndrome
- Beckwith-Wiedemann syndrome
Additionally, specific genetic mutations and chromosomal abnormalities have been linked to rhabdomyosarcoma development. One of the most well-known genetic alterations is the PAX/FOXO1 fusion gene, which is found in a significant proportion of alveolar rhabdomyosarcoma cases, a particularly aggressive subtype.
Environmental Factors
While genetic factors are considered the primary contributors to rhabdomyosarcoma development, environmental factors may also play a role. Some studies have suggested that exposure to certain chemicals, radiation, or viral infections during pregnancy or early childhood may increase the risk of developing rhabdomyosarcoma, but more research is needed to establish a clear causal relationship.
Diagnosis of Rhabdomyosarcoma
The diagnostic process for rhabdomyosarcoma typically involves several steps:
Imaging Tests: Imaging techniques such as X-rays, computed tomography (CT) scans, magnetic resonance imaging (MRI), and positron emission tomography (PET) scans are used to locate and evaluate the extent of the tumor.
Biopsy: A biopsy is performed to obtain a sample of the tumor tissue for further analysis. This can be done through a surgical procedure (part or all of the tumor may be surgically removed for analysis) or, in some cases, using a fine needle aspiration technique (a hollow needle is used to extract a tissue sample).
Histopathological Examination: A pathologist examines the biopsy sample under a microscope to determine the type of cells present and confirm the diagnosis of rhabdomyosarcoma.
Immunohistochemistry: Specific markers, such as desmin, myogenin, and MyoD1, are used to identify the muscle cell origin of the tumor.
Genetic Testing: Molecular testing is performed to identify specific genetic alterations, such as the PAX/FOXO1 fusion gene, which can help determine the subtype of rhabdomyosarcoma and guide treatment decisions.
Staging: Once the diagnosis is confirmed, additional tests, such as bone scans, CT scans, or PET scans, may be performed to determine the stage of the cancer, which indicates how far it has spread.
Watch a video about ultrasound-guided biopsy for soft tissue sarcoma
Treatment Options
The treatment of rhabdomyosarcoma typically involves a multidisciplinary approach, combining various modalities such as surgery, chemotherapy, and radiation therapy. The specific treatment plan is tailored to the individual patient based on factors like the subtype, stage, age, and tumor location.
Surgery
Surgery is often the first line of treatment for rhabdomyosarcoma, to remove as much of the tumor as possible. The extent of surgery depends on the size and location of the tumor, as well as the potential for preserving function and minimizing long-term side effects. In some cases, particularly for tumors located in sensitive areas like the head or neck, surgery may not be possible or may be limited to a biopsy procedure to obtain a tissue sample for diagnosis. Based on timing it could be upfront surgery (performed before chemotherapy/radiation for localized, resectable tumors) and delayed surgery (after chemotherapy and/or radiation to shrink the tumor and improve resectability).
Chemotherapy
Chemotherapy plays a crucial role in the treatment of rhabdomyosarcoma, both before and after surgery. It is typically administered in combination with radiation therapy, an approach known as chemoradiotherapy. The specific chemotherapy regimen may vary depending on the subtype and risk group of the patient.
Standard Regimens
1.VAC (Vincristine, Actinomycin D, and Cyclophosphamide)
- Used in North America for over 40 years as the standard chemotherapy regimen
- Given in combination with radiation therapy and/or surgery
- Typically administered every 3-4 weeks for 9-12 cycles
2. IVA (Ifosfamide, Vincristine, and Actinomycin D)
- Standard regimen used in Europe and some other countries
- Ifosfamide is substituted for cyclophosphamide in the VAC regimen
- May have better outcomes compared to VAC, but with increased toxicity
3. Intensified/High-Dose Regimens
- Used for high-risk or metastatic disease
- This may include higher doses of chemotherapy drugs or additional agents like doxorubicin, irinotecan, or topotecan
- Stem cell rescue (autologous stem cell transplant) may be used to support high-dose chemotherapy
4. Maintenance Chemotherapy
- Low-dose vinorelbine and cyclophosphamide given after standard chemotherapy
- Shown to improve survival rates in high-risk patients in the European rhabdomyosarcoma studies
- Radiation Therapy
Radiation therapy is often used in combination with chemotherapy, either before or after surgery, to target and destroy any remaining cancer cells. The type of radiation therapy used (external beam radiation or brachytherapy) and the dose depends on the location and extent of the tumor, as well as the patient’s age and overall health. In recent years, there has been a focus on reducing radiation exposure, particularly in children, to minimize the risk of long-term side effects and secondary cancers. This has led to the development of more targeted and precise radiation techniques, such as intensity-modulated radiation therapy (IMRT) and proton beam therapy.
5. External Beam Radiation Therapy (EBRT)
- Most common type of radiation used.
- Delivers high-energy beams from outside the body to the tumor site.
- Techniques like intensity-modulated radiation therapy (IMRT) and proton therapy help minimize radiation exposure to healthy tissues.
6. Brachytherapy
- Involves placing radioactive sources directly into or near the tumor.
- It may be used for certain tumor locations, like the vagina or bladder.
Timing
Adjuvant radiation: After surgery to target any remaining cancer cells.
Neoadjuvant radiation: Before surgery to shrink the tumor.
Concurrent with chemotherapy: Radiation given during chemotherapy cycles.
Emerging Therapies
Targeted Therapies
Anaplastic lymphoma kinase (ALK) inhibitors: For tumors with ALK gene alterations.
Anti-angiogenic agents: Targeting blood vessel formation (e.g., bevacizumab).
mTOR inhibitors: Targeting the mTOR pathway (e.g., temsirolimus, everolimus).
MEK inhibitors: Targeting the RAS/MAPK pathway (e.g., trametinib).
Immunotherapies
Immune checkpoint inhibitors: Blocking proteins like PD-1/PD-L1 to enhance anti-tumor immune response.
Cancer vaccines: Stimulating the immune system to recognize and attack cancer cells.
Adoptive cell therapies: Engineering a patient’s immune cells (e.g., CAR T-cells) to target cancer cells.
Other Novel Approaches
Oncolytic viruses: Viruses engineered to infect and kill cancer cells selectively.
Antibody-drug conjugates: Monoclonal antibodies linked to cytotoxic drugs for targeted delivery.
Risk-Adapted Treatment Protocols
In recent years, there has been a shift towards risk-adapted treatment protocols for rhabdomyosarcoma, which aim to tailor the intensity of treatment based on the individual patient’s risk profile. This approach aims to maximize the chances of cure while minimizing the long-term side effects of treatment, particularly in low-risk patients. Risk stratification is typically based on factors such as the subtype, stage, age, tumor location, and the presence of specific genetic alterations like the PAX/FOXO1 fusion gene. Patients with low-risk disease may receive less intensive treatment, while those with high-risk disease may receive more aggressive therapy, including higher doses of chemotherapy and radiation.
Ongoing clinical trials
Early Use of Vinorelbine: Phase III trial evaluating 24 weeks of vincristine and dactinomycin for very low-risk rhabdomyosarcoma, and examining centralized molecular risk stratification and therapy intensification for patients with DNA mutations.
Pediatric MATCH Trial: Phase II trial testing the ALK/ROS1 inhibitor ensartinib in solid tumors, including rhabdomyosarcoma, with ALK or ROS1 alterations.
Cabozantinib-S-Malate: Phase II trial evaluating this multi-kinase inhibitor in recurrent, refractory or newly diagnosed sarcomas and rare tumors, including rhabdomyosarcoma.
Combination Chemotherapy +/- Temsirolimus: Phase III trial comparing standard chemotherapy alone versus chemotherapy plus the mTOR inhibitor temsirolimus in intermediate-risk rhabdomyosarcoma.
Elimusertib Trial: Phase I/II trial testing the safety and efficacy of elimusertib, a novel agent targeting tumor cell growth, in relapsed/refractory solid tumors like rhabdomyosarcoma.
Prognosis
The stage of rhabdomyosarcoma at the time of diagnosis is a crucial factor in determining the prognosis. Early-stage tumors that are localized and have not spread are generally associated with better outcomes compared to advanced-stage tumors that have metastasized to other organs or distant sites.
Age and Prognosis
Age is another important factor in determining prognosis. Children and adolescents generally have better outcomes compared to adults with rhabdomyosarcoma. This may be due to differences in tumor biology, as well as the ability to tolerate more intensive treatment regimens.
Patient’s Survivorship
According to the American Cancer Society, the overall 5-year survival rate for rhabdomyosarcoma is approximately 70%. However, survival rates can vary significantly based on the factors mentioned above. For example, the 5-year survival rate for localized embryonal rhabdomyosarcoma in children is around 90%, while the rate for metastatic alveolar rhabdomyosarcoma in adults is much lower, around 20%.
Early diagnosis and prompt treatment are crucial for improving survival outcomes, as they increase the chances of catching the cancer at an earlier stage when it is more treatable. It is worth mentioning that during and after treatment, patients may face a range of problems or side effects, which require careful management and follow-up.
During Treatment
Side Effects of Chemotherapy
- Nausea, vomiting, fatigue, hair loss.
- Increased risk of infections due to low blood counts (neutropenia).
- Potential long-term effects like heart problems and infertility.
Side Effects of Radiation Therapy
- For radiation to the head/brain area: headaches, memory loss, personality changes, trouble learning.
- Formation of scar tissue in radiated areas.
- Slowing of bone growth and potential deformities or stunted growth in children, depending on age and areas exposed to radiation.
After Treatment
Short-Term Issues
- Emotional distress, anxiety about cancer recurrence.
- School/learning difficulties due to missed time or effects of treatment.
Long-Term Issues
- Risk of second cancers later in life from chemotherapy and radiation exposure.
- Heart problems (cardiomyopathy) after certain chemotherapy drugs like doxorubicin.
- Reproductive/fertility issues from chemotherapy or radiation.
- Bone/growth problems if radiation was given to certain areas during childhood.
- Dental problems from chemotherapy and radiation.
- Endocrine (hormone) disorders like thyroid dysfunction.
Careful long-term follow-up and survivorship care is crucial to monitor for potential late effects and provide appropriate management. Multidisciplinary support services are often needed to address the various medical, psychosocial, and developmental needs of rhabdomyosarcoma survivors. While modern treatment approaches have improved survival rates, the intensive multimodality therapy required can lead to significant acute and long-term complications, necessitating proactive monitoring and interventions to optimize the quality of life for rhabdomyosarcoma patients and survivors.
What Patients Should Do
Follow-up Care
- Attend regular follow-up appointments with the cancer care team for physical exams, imaging tests (CT, MRI, PET scans), and lab tests to monitor for any recurrence or late effects.
- Follow-up visits will be more frequent initially (e.g. every 3-6 months) but may decrease over time if there are no issues.
- Routine monitoring for physical growth, sexual development, bladder function, vision/eye exams (if radiation was given to the head/eye area), and dental exams is crucial, especially in children.
Survivorship Care Plan
- Discuss developing a survivorship care plan with the treatment team, including a summary of treatment received, schedule for follow-up care, screening recommendations for other cancers, and information on possible late effects.
- Keep detailed personal medical records documenting the cancer history to facilitate long-term follow-up care as the child transitions to adult care.
Rehabilitation Services
- Utilize rehabilitation services like physical therapy, occupational therapy, counseling, nutritional support, and educational assistance to regain function and cope with the effects of cancer and its treatment.
- Cancer rehabilitation aims to help survivors and families regain control over various aspects of their lives.
Emotional/Psychosocial Support
- Seek emotional and psychosocial support if needed to cope with anxiety, depression, cognitive/learning issues, or other effects on development and relationships.
- Mental health professionals and support groups can help address emotional challenges faced by survivors and families.
Monitoring for Late Effects
- Be vigilant for potential long-term side effects like heart/lung problems, growth abnormalities, infertility, and second cancers based on the specific treatment received.
- Follow-up care should address quality-of-life concerns, including developmental, cognitive, or emotional issues
- Consistent follow-up care, adherence to survivorship care recommendations, rehabilitation services, psychosocial support, and proactive monitoring/management of late effects are crucial for optimizing long-term outcomes and quality of life for rhabdomyosarcoma survivors.
Watch 14-year-old Kasey’s touching story
Conclusion
While rhabdomyosarcoma is an aggressive childhood cancer, there are reasons for hope. Modern multimodal treatment approaches have significantly improved overall survival rates. Ongoing research evaluating promising new targeted therapies and immunotherapies offers the potential for further enhancing cure rates while reducing treatment toxicities. With access to specialized multidisciplinary care, adherence to protocols, consistent follow-up, and supportive services, many patients can overcome this challenge. The resilience of children and loved ones, combined with dedicated research efforts, inspires this difficult battle. Though the journey is arduous, the future remains encouraging as advances continue for better outcomes against rhabdomyosarcoma.
Resources
- Clinicaltrials.gov
- American Society of Clinical Oncology (ASCO) – Cancer.net
- National Cancer Institute – cancer.gov
- Children’s Hospital of Philadelphia – Chop.edu
- American Cancer Society – cancer.org
- Yale Medicine – yalemedicine.org
- Rhabdomyosarcoma – PubMed
- Penn Medicine Abramson Cancer Center – pennmedicine.org
- Memorial Sloan Kattering Cancer Center – mskcc.org
- Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma – PubMed
- Current and Future Treatment Strategies for Rhabdomyosarcoma – PubMed
- University of California San Francisco UCSF Clinical Trials – clinicaltrials.ucsf.edu
- Canadian Cancer Society – cancer.ca
- Oncodaily.com – Everything related to cancer such as everyday news, blogs, videos, podcasts, etc.


