
Gastrinoma։ What patients should know about
What Is Gastrinoma?
Gastrinoma is a rare type of tumor that forms in the pancreas or small intestine and makes too much of a hormone called gastrin. Gastrin’s normal job is to tell the stomach to make acid that helps digest food. But when the body makes too much gastrin, it causes the stomach to produce too much acid, which can lead to serious digestive problems.
This overproduction of stomach acid can cause painful ulcers, diarrhea, and other symptoms. The condition caused by a gastrinoma is known as Zollinger-Ellison Syndrome (ZES). People with ZES may have ulcers that don’t heal or keep coming back, even when they take medicine.Gastrinomas are part of a group of tumors called neuroendocrine tumors. These are growths that form from hormone-producing cells. Some gastrinomas happen on their own, but others may be part of a genetic condition called Multiple Endocrine Neoplasia Type 1 (MEN1). In MEN1, people can have tumors in more than one hormone-producing gland, including the pancreas, parathyroid, and pituitary glands. Understanding what gastrinoma is and how it works in the body is the first step to managing the condition and getting the right care.
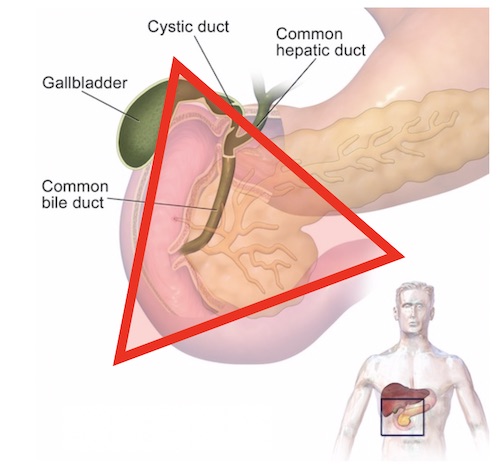
The majority of Gastrinomas are found within this triangle first described by Dr. Stabile and colleagues in 1984. This image is taken from operativereview.com
What Causes Gastrinoma?
Most cases of gastrinoma happen sporadically, meaning they develop by chance and are not inherited. These tumors can form in people with no family history or known risk factors, and they are the most common type.
However, in about 20–25% of cases, gastrinomas are part of a genetic condition called Multiple Endocrine Neoplasia Type 1 (MEN1). This condition causes tumors to grow in several hormone-producing glands, including the pancreas, parathyroid glands, and pituitary gland. If a person has MEN1, there is a 50% chance they could pass it on to their children. The cause of gastrinoma in MEN1 is a mutation (change) in the MEN1 gene, which normally helps control how cells grow. When this gene is not working properly, it can lead to uncontrolled cell growth and tumor development in hormone-producing glands.
People who are at higher risk of developing gastrinoma include:
- Those with a family history of MEN1
- Individuals with other MEN1-related tumors
- People typically between the ages of 30 and 50
- Men may be slightly more affected than women in sporadic cases
Understanding whether a gastrinoma is inherited or not is important because it can guide genetic testing, screening for family members, and long-term care planning.
Common Symptoms and Early Signs
Gastrinomas can cause a variety of symptoms, most of which are linked to the extra acid being produced in the stomach. These symptoms can sometimes be mistaken for more common digestive issues, so it’s important to recognize the warning signs. One of the main symptoms is severe, recurring peptic ulcers. These are sores in the lining of the stomach or small intestine that can cause burning pain, especially between meals or at night. Unlike typical ulcers, these may not heal well with standard treatments and can come back again and again. Other common symptoms include:
- Abdominal pain, especially in the upper belly
- Nausea and vomiting, sometimes with blood if ulcers are severe
- Diarrhea, which may be frequent or watery
- Weight loss, even without trying
- Bloating and discomfort after eating
- Heartburn or acid reflux that doesn’t improve with regular antacids
These symptoms are all signs of Zollinger-Ellison Syndrome (ZES), the condition caused by high gastrin levels from a gastrinoma. In ZES, the stomach becomes overly acidic, which leads to many of these digestive problems. If you or someone you know has any of these signs—especially repeated ulcers or long-lasting heartburn—it’s important to talk to a doctor. Early diagnosis and treatment can help prevent serious complications.
How Is Gastrinoma Diagnosed?
Diagnosing gastrinoma usually involves a combination of blood tests, imaging scans, and sometimes a biopsy. These tests help doctors confirm that too much gastrin is being made and locate any tumors in the body. One of the most important tests is a fasting serum gastrin blood test. This measures the amount of gastrin hormone in your blood after you haven’t eaten for several hours. If the levels are very high, it may be a sign of gastrinoma. Doctors also check your gastric pH, which tells how acidic your stomach is. A very low pH (more acid) combined with high gastrin levels is a strong clue that a gastrinoma might be present.
Sometimes, a secretin stimulation test is used to confirm the diagnosis. In this test, doctors give you a small amount of a substance called secretin through an IV. In people with gastrinoma, this causes a big jump in gastrin levels, while in others it does not. To find the tumor, doctors use imaging tests like:
- CT scans or MRI to see inside your body
- Endoscopic ultrasound, where a thin camera is passed into your stomach to get detailed images
- Ga-68 DOTATATE PET scan, a special type of scan that looks for neuroendocrine tumors by detecting hormone-related signals
In some cases, a biopsy may be needed. This means removing a small piece of the tumor tissue to examine it under a microscope and confirm it’s a gastrinoma.
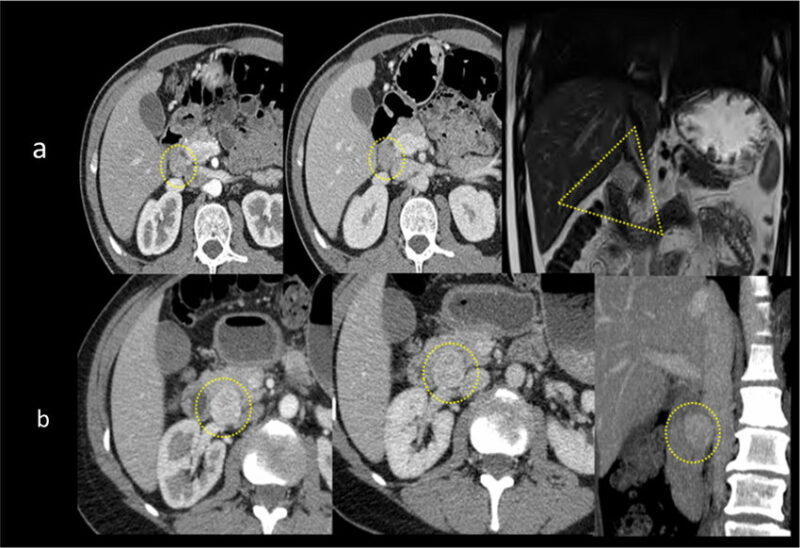
Frequent locations of pNETs: anatomical imaging. A gastrinoma is typically located in the “gastrinomas triangle” (yellow dotted triangle). Extrapancreatic nodule measuring 10 mm located in the gastrinomas triangle with homogeneous arterial (right), and portal contrast enhancement (middle). b Insulinoma is more often localized in the pancreas head. This case shows a 25-mm nodule with arterial (right), and portal contrast hyperenhancement (middle). The report should always include the absence of vascular and perineural involvements (as well as distal metastases) in order to confirm the resectability. This image is taken from researchgate.net
Treatment Options for Gastrinoma
Treating gastrinoma focuses on controlling stomach acid, removing tumors when possible, and keeping hormone levels in check. The treatment plan depends on the size and location of the tumor and whether it has spread (metastasized).
The first step for many patients is taking medications to reduce acid. These are called proton pump inhibitors (PPIs)and include drugs like omeprazole or lansoprazole. They help lower the amount of acid the stomach makes, which can relieve symptoms like ulcers, pain, and heartburn. If the tumor is found early and is localized (hasn’t spread), doctors may recommend surgery to remove it. Surgery offers the best chance for a cure in these cases. For tumors that can’t be removed or have spread to other parts of the body, other treatments are used:
- Somatostatin analogs (like octreotide or lanreotide) help slow hormone production and control symptoms like diarrhea or pain.
- Targeted therapy or chemotherapy may be used for metastatic gastrinomas, especially if they grow quickly or cause complications.
Patients with gastrinoma need regular monitoring, including blood tests to check gastrin levels and imaging scans to track tumor growth or new tumors. With the right treatment and ongoing care, many people with gastrinoma can manage their condition well and maintain a good quality of life.
Living with Gastrinoma: What to Expect
Living with gastrinoma can be challenging, especially if the tumor has spread or cannot be completely removed. In these cases, the condition may become chronic, meaning it needs long-term management rather than a one-time cure.
Regular follow-up care is very important. This includes routine blood tests to monitor hormone levels, imaging scans to check for tumor growth, and regular visits with your healthcare team. Staying on top of these check-ups helps catch any changes early and adjust treatment as needed. Many people manage symptoms through a combination of medications and lifestyle changes. Taking proton pump inhibitors (PPIs) as prescribed keeps stomach acid under control. In some cases, changing your diet—such as avoiding spicy or acidic foods—can also help reduce symptoms like heartburn or discomfort.
Don’t forget the emotional side of living with a rare condition. It’s normal to feel overwhelmed at times. Talking to a mental health counselor, joining a support group, or connecting with others who have neuroendocrine tumors can provide comfort and guidance. With the right care, many people with gastrinoma are able to live active, full lives while managing their condition.
Genetic Testing and Family Risk
In some cases, gastrinoma is linked to a genetic condition called Multiple Endocrine Neoplasia Type 1 (MEN1). When a person is diagnosed with gastrinoma as part of MEN1, their close family members may also be at risk, since MEN1 can be inherited.
This is why genetic testing is often recommended for first-degree relatives—like parents, siblings, and children—of someone with MEN1-related gastrinoma. A simple blood test can check for changes in the MEN1 gene, which helps doctors determine who else in the family may be at risk of developing similar tumors. Early detection is extremely important in hereditary cases. If doctors know someone carries the MEN1 gene mutation, they can begin screening and monitoring even before symptoms appear. This can lead to early treatment, better outcomes, and less risk of complications.
Genetic counseling plays a key role in this process. A genetic counselor helps families understand what the test results mean, talks through the risks and options, and provides support as they make decisions about testing and care. Knowing about MEN1 in the family gives everyone a chance to take charge of their health and plan ahead with confidence.
Myths and Misconceptions About Gastrinoma
Because gastrinoma is rare, there are several misunderstandings about what it is and how it affects the body. Clearing up these myths can help patients and families feel more informed and confident about managing the condition.
- It’s just a bad ulcer: While gastrinoma causes ulcers, it’s much more than that. These ulcers are the result of a tumor that produces too much gastrin, leading to excess acid in the stomach. Regular ulcer treatments like antacids often don’t work well, and the real cause—the tumor—needs to be addressed.
- All gastrinomas are cancerous: Not all gastrinomas are cancerous (malignant). Some can be benign, meaning they don’t spread to other parts of the body. However, about 50–60% of gastrinomas can become malignant, especially if left untreated. That’s why it’s important to diagnose and monitor them early.
- Surgery cures everything: Surgery can be very helpful—especially if the tumor is caught early and hasn’t spread—but it may not be a complete cure, especially in advanced cases. Some people may still need long-term medication or treatment to control acid production or manage symptoms.
Even after treatment, ongoing care is essential. Gastrinoma can come back or cause new symptoms, so regular check-ups, hormone monitoring, and imaging tests are important to stay healthy in the long run.
To learn more, including common myths and misconceptions, visit the OncoDaily YouTube TV
FAQ
What is a gastrinoma?
A gastrinoma is a rare tumor, usually in the pancreas or small intestine, that produces too much of the hormone gastrin, causing excess stomach acid.
What condition is caused by gastrinoma?
Gastrinoma causes a condition called Zollinger-Ellison Syndrome (ZES), which leads to severe ulcers, acid reflux, and diarrhea.
Is gastrinoma cancerous?
Not always. Some gastrinomas are benign, but around 50–60% can become malignant, especially if untreated.
What are the early symptoms of gastrinoma?
Common symptoms include abdominal pain, recurring ulcers, diarrhea, nausea, weight loss, and acid reflux.
How is gastrinoma diagnosed?
Diagnosis involves blood tests (gastrin levels), gastric pH testing, secretin stimulation test, and imaging scans like CT, MRI, or PET.
What is the gastrinoma triangle?
It’s the area where most gastrinomas are found—between the pancreas, duodenum, and bile ducts.
What treatments are available for gastrinoma?
Treatments include proton pump inhibitors (PPIs) to reduce acid, surgery if tumors are removable, and other medications like somatostatin analogs or chemotherapy.
Is gastrinoma hereditary?
It can be. About 20–25% of cases are linked to Multiple Endocrine Neoplasia Type 1 (MEN1), a genetic condition.
Should family members of someone with MEN1-related gastrinoma get tested?
Yes. Genetic testing is recommended for first-degree relatives to detect MEN1 mutations early.
Can people live a normal life with gastrinoma?
Yes. With proper treatment, monitoring, and lifestyle changes, many people live full, active lives.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
