
FDA Approved Pluvicto (177Lu-PSMA-617) for Metastatic Castration-Resistant Prostate Cancer
On March 28, 2025, the U.S. Food and Drug Administration (FDA) expanded the approval of Pluvicto (177Lu-PSMA-617) for treating metastatic castration-resistant prostate cancer (mCRPC). This decision marks a major milestone in the management of advanced prostate cancer, particularly for patients who have limited treatment options.
Pluvicto, a radioligand therapy (RLT), is now authorized for earlier-line treatment in patients with prostate-specific membrane antigen (PSMA)-positive mCRPC, including those who have not yet received chemotherapy. This expanded approval is based on groundbreaking clinical trials demonstrating its efficacy in improving progression-free survival (PFS) and overall survival (OS) in patients with advanced disease.
What is Pluvicto?
Pluvicto is a targeted radioligand therapy designed to deliver a lethal dose of radiation directly to PSMA-expressing cancer cells while sparing surrounding healthy tissue. The treatment consists of Lutetium-177 (177Lu), a beta-emitting radioactive isotope, bound to a PSMA-targeting small molecule. Once injected, it binds to PSMA-positive tumors, delivering radiation selectively to cancer cells, reducing tumor burden, and prolonging survival.
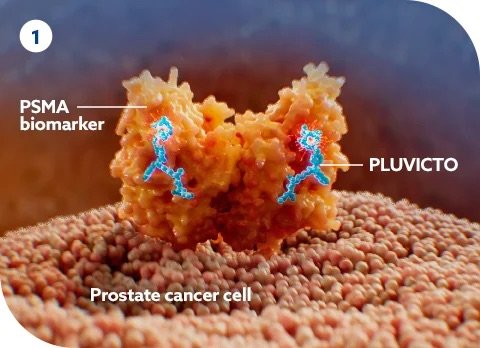
How Does 177Lu-PSMA-617 Work?
Pluvicto is a cutting-edge treatment for metastatic castration-resistant prostate cancer (mCRPC), designed to specifically target and destroy cancer cells while minimizing harm to healthy tissue. Here’s a breakdown of how Pluvicto works to fight prostate cancer:
- Targeting Prostate Cancer Cells:The drug consists of a molecule that binds to PSMA, a cell surface protein found in high amounts on prostate cancer cells, especially in cases of metastatic castration-resistant prostate cancer (mCRPC).
- Delivery of Radioactive Treatment:Once Pluvicto binds to the PSMA on the cancer cells, it delivers a dose of beta radiation directly to those cells. This radiation has a localized effect, causing significant DNA damage within the cancer cells
- Cancer Cell Death:The radiation from Pluvicto leads to damage in the cancer cell’s DNA, which disrupts the cell’s ability to replicate and survive. This ultimately results in cell death and the shrinking or elimination of cancer cells.
- Sparing Healthy Tissue:Since Pluvicto specifically targets cells with PSMA, healthy cells that do not express this antigen are largely spared from the radiation, minimizing damage to surrounding tissues. This targeted mechanism is a major advantage over conventional chemotherapy, which affects both cancerous and healthy cells.
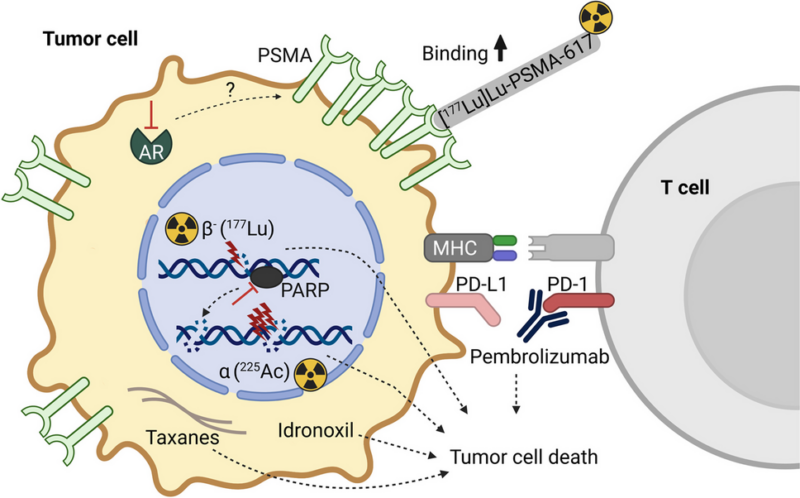
Efficacy and Safety
Efficacy was evaluated in PSMAfore (NCT04689828), a randomized, multicenter, open-label trial enrolling 468 patients with PSMA-positive mCRPC and progression on one ARPI, who the investigator considered appropriate for delay of taxane-based chemotherapy. Patients were randomized (1:1) to receive lutetium Lu 177 vipivotide tetraxetan (7.4 GBq [200 mCi] every 6 weeks for 6 doses) or a change in ARPI. Patients who progressed on the ARPI arm were allowed to crossover to the experimental therapy.
The major efficacy outcome was radiographic progression-free survival (rPFS) by blinded independent central review. Overall survival (OS) was an additional efficacy outcome. Median rPFS was 9.3 months (95% confidence interval [CI]: 7, not estimable) in the lutetium Lu 177 vipivotide tetraxetan arm and 5.6 months (95% CI: 4, 6) in the ARPI arm. Median OS was 24.5 months (95% CI: 19.5, 28.9) and 23.1 months (95% CI: 19.6, 25.5) in the respective arms.
The recommended lutetium Lu 177 vipivotide tetraxetan dose is 7.4 GBq (200 mCi) intravenously every 6 weeks for 6 doses, or until disease progression or unacceptable toxicity.
KEY trials for FDA approval
The expanded Pluvicto approval was based on several high-impact clinical trials, including PSMAfore and VISION, which demonstrated significant benefits in earlier lines of therapy.
PSMAfore Trial
The PSMAfore Trial (NCT04689828) marks a pivotal moment in the treatment evolution of metastatic castration-resistant prostate cancer (mCRPC). This Phase 3 clinical trial evaluated Pluvicto (lutetium Lu 177 vipivotide tetraxetan), a PSMA-targeting radioligand therapy, in patients who had not previously undergone chemotherapy. The goal was to compare its efficacy and safety to standard androgen receptor pathway inhibitors (ARPIs) such as enzalutamide and abiraterone.Patients were randomized to receive either Pluvicto or a change in ARPI therapy.
Pluvicto significantly outperformed ARPIs, demonstrating a median rPFS of 12.0 months, compared to 5.6 months in the ARPI group. This underscores Pluvicto’s ability to effectively delay disease progression.The ORR was markedly higher in the Pluvicto group, reaching 50.7% versus 14.9% in those treated with ARPIs.A ≥50% decline in prostate-specific antigen (PSA) levels was seen in 57.6% of patients treated with Pluvicto, versus 20.4% of those on ARPIs. PSA response is a strong indicator of therapeutic efficacy in mCRPC (American Cancer Society).
VISION Trial
The VISION Trial (NCT03511664) was the pivotal Phase 3 study that led to the initial FDA approval of Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for patients with metastatic castration-resistant prostate cancer (mCRPC) who had previously been treated with both androgen receptor pathway inhibitors (ARPIs) and taxane-based chemotherapy. This global, randomized study provided compelling evidence of Pluvicto’s efficacy and safety in a heavily pretreated population.
In terms of overall survival (OS), Pluvicto significantly extended survival time. Patients who received Pluvicto had a median OS of 15.3 months, compared to 11.3 months in the standard care group. This represented a 38% reduction in the risk of death.Pluvicto also showed marked improvement in radiographic progression-free survival (rPFS). Median rPFS was 8.7 months with Pluvicto versus 3.4 months in the control group.
In terms of prostate-specific antigen (PSA) response, 46.0% of patients in the Pluvicto group experienced a ≥50% decline in PSA levels, compared to only 7.1% in the standard care group.Safety and tolerability were consistent with expectations for radioligand therapy. The most common side effects reported were fatigue (43%) and dry mouth (38%). Grade 3 or 4 adverse events occurred in 52.7% of patients in the Pluvicto arm, compared to 38.0% in the control group(New England Journal of Medicine (NEJM)).
For years, treatment for mCRPC primarily relied on chemotherapy and androgen receptor inhibitors with limited durability; today, Pluvicto offers a new era of precision-targeted radioligand therapy—delivering not only deeper responses but also renewed hope for patients earlier in their treatment journey.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
