A cell becomes cancerous when something goes wrong with its DNA. Normally, DNA works like an instruction book, telling the cell when to grow, when to divide, and when to stop. If those instructions are broken or not followed correctly, the cell can lose control and start dividing without limits—that’s when we call it cancer. But DNA can go wrong in more than one way. Sometimes the letters of the code itself are damaged—this is called a mutation. Other times, the DNA sequence is still perfectly fine, but the cell is reading the book incorrectly. This happens because of changes in how the DNA is packaged and marked inside the cell. These changes are called epigenetic modifications.

Photo: Depositphotos
Unlike mutations, epigenetic changes don’t rewrite the DNA—they just flip the switches that decide whether a gene is ON or OFF. In cancer, these switches can be misused: important protective genes get silenced, while harmful ones stay active. The exciting part is that scientists are learning how to flip those switches back. This new strategy, known as epigenetic therapy, is opening the door to treatments that reprogram cancer cells rather than simply trying to kill them. Shikhar Sharma, Theresa K. Kelly, Peter A. Jones, Epigenetics in cancer, Carcinogenesis, Volume 31, Issue 1, January 2010, Pages 27–36.
This article explores epigenetic modifiers—drugs that target abnormal gene switches in cancer cells. Unlike traditional therapies, they don’t damage DNA directly but reprogram how genes are turned ON or OFF, offering new ways to restore normal growth control and block cancer progression.
The Role of Epigenetics in Cancer
In cancer, this balance is lost. Sometimes the DNA code itself is mutated. But other times, the epigenetic switches that control the genes are flipped the wrong way. For example, a brake gene that normally stops uncontrolled growth might get silenced by DNA methylation.
What makes this especially dangerous is that epigenetic changes can spread across many genes at once, reprogramming the entire behavior of a cell. The result is a cell that no longer listens to the body’s rules—it divides uncontrollably, ignores signals to die, and sometimes even hides from the immune system.
Scientists now know that abnormal epigenetic patterns are found in nearly all types of cancer. That’s why researchers are working on drugs that can reset these switches, giving the body back its control over dangerous cells. Yu, X., Zhao, H., Wang, R. et al. Cancer epigenetics: from laboratory studies and clinical trials to precision medicine. Cell Death Discov. 10, 28 (2024).
Traditional cancer treatments, like chemotherapy or radiation, mainly try to kill fast-dividing cells. The problem is, they often harm healthy cells too. Epigenetic therapy is different. Instead of attacking the DNA directly or breaking it apart, these drugs reprogram cancer cells by changing the way their genes are “read.”
How It Works?
Every cell in the body has the same DNA, but not all genes are active. Some are “on,” while others are “off.” Cancer often develops when important protective genes, known as tumor suppressors, are switched off, or when harmful genes, called oncogenes, are switched on. Epigenetic drugs work by restoring balance: they can turn tumor suppressor genes back on so they can stop cancer growth, and switch oncogenes off so they can no longer drive cancer forward.
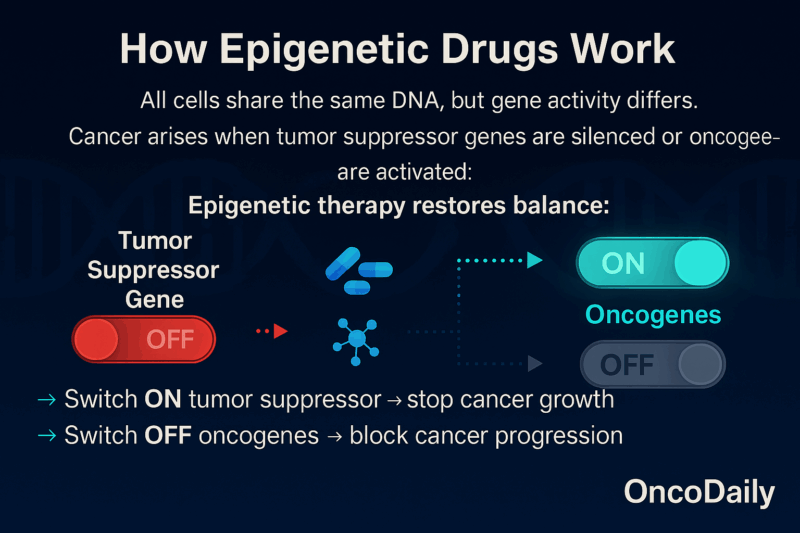
This is not about changing the DNA sequence. Instead, it’s about changing the instructions the cell follows. Think of it like fixing the software without rewriting the hardware. Bouyahya A, The Role of Epigenetic Modifications in Human Cancers and the Use of Natural Compounds as Epidrugs: Mechanistic Pathways and Pharmacodynamic Actions. Biomolecules. 2022 Feb
How Epigenetic Therapy Works in Practice
When people first hear about epigenetic therapy, one of their biggest questions is: “What will this treatment actually be like for me?” Unlike chemotherapy or radiation, which directly destroy cancer cells, epigenetic drugs work by reprogramming them. They do not change the DNA itself, but they reset the “switches” that control which genes are active and which are silent. In many cancers, protective genes are switched off and harmful genes are switched on. Epigenetic therapy helps restore the normal balance so that the cancer grows more slowly, responds better to other treatments, or becomes more visible to the immune system.
Epigenetic changes are like little tags and signals placed on our DNA or the proteins around it. These tags don’t change the genetic letters themselves, but they decide whether certain parts of the DNA can be read or ignored. The two main types in cancer are:
DNA Methylation – the “Off Switch”
Imagine your DNA as a book, and every gene is a chapter. Adding a methyl tag (a tiny chemical group) is like putting a piece of tape over the chapter—you can’t open and read it. In cancer, protective chapters (tumor suppressor genes) often get taped shut, so the cell loses its safety checks.
DNA methylation is characterized by the addition of a methyl group to the C5 position of cytosine residues by a DNA methyltransferase (DNMT). The resulting epigenetic methylation groups differentiate normal cells from cancerous and other diseased cells. The human genome encodes three conserved DNMT subtypes: DNMT1, DNMT3A, and DNMT3B. DNMT plays dual roles in tumor drug resistance. The mRNA expression levels of DNMT show diametrically opposed sensitivity to inhibitors in different tumors. For example, DNMT3B inhibition significantly increases the sensitivity of the inhibitor in pancreatic cancer, while ovarian cancers with high DNMT expression of are more sensitive to inhibitor treatment.
Histone Modifications – Packaging the Book
DNA is wrapped around proteins called histones, like thread around spools. If the wrapping is tight, the gene is hidden and cannot be read. If the wrapping is loose, the gene is exposed and active. Chemical tags can make histones tighten or loosen, controlling gene activity. In cancer, this packaging often gets messed up, hiding the wrong genes or activating harmful ones.
In practical terms, epigenetic therapy is usually given either as a pill that can be taken at home or as an infusion through a vein at the hospital. The exact schedule depends on the drug and the type of cancer, but patients typically receive it in cycles — for example, several days of treatment followed by a rest period. This cycle may repeat for months. Doctors monitor progress through regular blood tests and scans, because it can take some time before the effects of the therapy are visible. Unlike chemotherapy, where changes may be rapid and sometimes harsh, epigenetic therapy often works more gradually.
At present, these drugs are mainly used for blood cancers such as certain leukemias, lymphomas, and myelodysplastic syndromes. However, many clinical trials are exploring their benefits in solid tumors like breast, lung, and colon cancer. Sometimes epigenetic therapy is given alone, but more often it is combined with other treatments. For example, when used alongside chemotherapy, it can make cancer cells more sensitive to the drug. When paired with immunotherapy, it may help the immune system recognize and attack the tumor more effectively.
For daily life, patients should expect regular hospital visits for infusions or check-ups, as well as frequent blood tests. The treatment requires patience, because results are often seen only after several cycles. But for many, the gentler nature of epigenetic therapy allows them to continue more of their normal activities compared to harsher cancer treatments.
What Drugs Are Used and How Often?
Several epigenetic drugs have already been approved by regulatory agencies like the US FDA, EMA, PMDA (Japan), and NMPA (China). They are available for different diseases and are taken at different schedules:
Chidamide (Tucidinostat, Epidaza)
Chidamide is an orally-available histone deacetylase inhibitor (HDACi) selective for several HDACs. Its action increases acetylation on histones, alters signaling pathways (e.g. PI3K/Akt and MAPK/Ras), and triggers cancer-cell death and growth arrest. It was approved by China’s regulatory authority (CFDA, now NMPA) in December 2014 for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL). Shi, Y., Jia, B., Xu, W. et al. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol 10, 69 (2017).
Vorinostat (Zolinza, SAHA)
Vorinostat was the first HDAC inhibitor approved in the U.S. for cancer treatment. It works by inhibiting HDACs (class I, II, and IV), which leads to accumulation of acetylated histones and proteins, allowing genes that suppress tumor growth to be turned ON. It was approved by the U.S. FDA on October 6, 2006 for the treatment of cutaneous T-cell lymphoma (CTCL) in patients with progressive, persistent, or recurrent disease.
Romidepsin (Istodax, FK228)
Romidepsin is another histone deacetylase inhibitor, more selective for class I HDACs. It exerts anti-cancer effects in CTCL and peripheral T-cell lymphoma (PTCL) by promoting apoptosis and affecting cell cycle and differentiation. It was approved by the FDA in November 2009 for CTCL. Later, it was FDA-approved in 2011 for PTCL in patients with at least one prior therapy. Robey RW, Chakraborty AR, Basseville A, Luchenko V, Bahr J, Zhan Z, Bates SE. Histone deacetylase inhibitors: emerging mechanisms of resistance. Mol Pharm. 2011 Dec 5;8(6):2021-31.
Belinostat (Beleodaq, PXD101)
Belinostat is a pan-HDAC inhibitor (affects HDAC class I, II, IV) with a hydroxamate structure. It is given intravenously. Its activity includes inducing durable responses in relapsed or refractory PTCL. It was approved by the FDA in July 2014 for the treatment of relapsed or refractory peripheral T-cell lymphoma.
Panobinostat (Farydak)
Panobinostat is a pan-HDAC inhibitor, meaning it inhibits multiple classes of HDACs. It has shown efficacy in multiple myeloma, especially in patients who have had prior treatments. It is used in combination with other drugs (e.g. bortezomib and dexamethasone) in that disease. It was approved by the FDA in February 2015 for treatment of relapsed or refractory multiple myeloma. The EMA (European Medicines Agency) also approved it for this indication. Zhang Q, Wang S, Chen J, Yu Z. Histone Deacetylases (HDACs) Guided Novel Therapies for T-cell lymphomas. Int J Med Sci 2019; 16(3):424-442. doi:10.7150/ijms.30154. https://www.medsci.org/v16p0424.htm
What Are the Benefits and Challenges of Epigenetic Drugs?
Developing and applying epigenetic drugs is far from straightforward. One of the greatest difficulties is specificity. Epigenetic modifications like DNA methylation or histone acetylation regulate thousands of genes at once. When a drug changes these marks, it does not only affect the cancer-driving genes but can also switch on or silence many unrelated genes. This broad activity increases the risk of unwanted side effects and toxicity, as normal cells may lose their balance too.
Another challenge lies in delivery. Epigenetic drugs must reach the right tissues, enter the nucleus, and interact with the correct enzymes, but many of them have poor stability or solubility in the body. This makes it difficult to ensure that the drug works exactly where it is needed, without spreading damage. Researchers are exploring nanoparticle-based delivery systems to make these therapies more precise.
A further complication is drug resistance. Cancer cells are highly adaptable: when one epigenetic pathway is blocked, they may activate another, or develop mutations that prevent the drug from binding to its target. This means that while patients might respond at first, the tumor can return in a more aggressive form. Combining epigenetic drugs with chemotherapy, immunotherapy, or targeted therapy is one strategy scientists are testing to overcome this problem.
Moreover, epigenetic changes are often reversible and dynamic. Unlike mutations, which are permanent, epigenetic marks can shift back and forth. This makes Side effects, although milder than chemotherapy, are still a concern. Fatigue, lowered blood counts, and higher risk of infection are common. For people who are already weak or have other illnesses, this can still be difficult.
Another challenge is that most of the proven success so far has been in blood cancers. For solid tumors like lung or breast cancer, research is ongoing, and while early results are promising, they are not yet as strong as in blood-related cancers. “Campbell RM, Tummino PJ. Cancer epigenetics drug discovery and development: the challenge of hitting the mark. J Clin Invest. 2014 Ja, Epub 2014 Jan 2. Erratum in: J Clin Invest. 2014 Mar
One of the main benefits is that epigenetic drugs are often gentler than chemotherapy or radiation. Because they do not attack all fast-growing cells, they usually cause fewer harsh side effects such as hair loss or severe nausea. Many patients are able to continue more of their daily activities during treatment. Another benefit is that these drugs can make other therapies work better.
For example, a cancer cell that had become resistant to chemotherapy may become sensitive again after epigenetic treatment. Similarly, by changing the way cancer cells are seen by the body, these drugs can make immunotherapy more effective. This ability to work in combination with other treatments is one of the strongest reasons doctors are hopeful about them.
You Can Also Read Tumor-Infiltrating Lymphocytes (TILs): How Our Immune System Enters the Fight Against Cancer by OncoDaily

Leading Cancer Organizations Support Epigenetic Therapy
These drugs have been approved by various regulatory bodies, such as the US FDA, the European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency (PMDA). They are used to treat conditions such as multiple myeloma (MM), cutaneous T-cell lymphoma (CTCL), and peripheral T-cell lymphoma (PTCL).
Additionally, chidamide (tucidinostat) was approved by PMDA in Japan and National Medical Products Administration (NMPA) in China for the treatment of PTCL and advanced breast cancer, and more recently, givinostat (ITF2357) was approved by the FDA in March 2024 as the first nonsteroidal treatment for Duchenne muscular dystrophy (DMD) for patients aged six years and older. Dai, W., Qiao, X., Fang, Y. et al. Epigenetics-targeted drugs: current paradigms and future challenges. Sig Transduct Target Ther 9, 332 (2024).
Written by Anahit Mkrtchyan