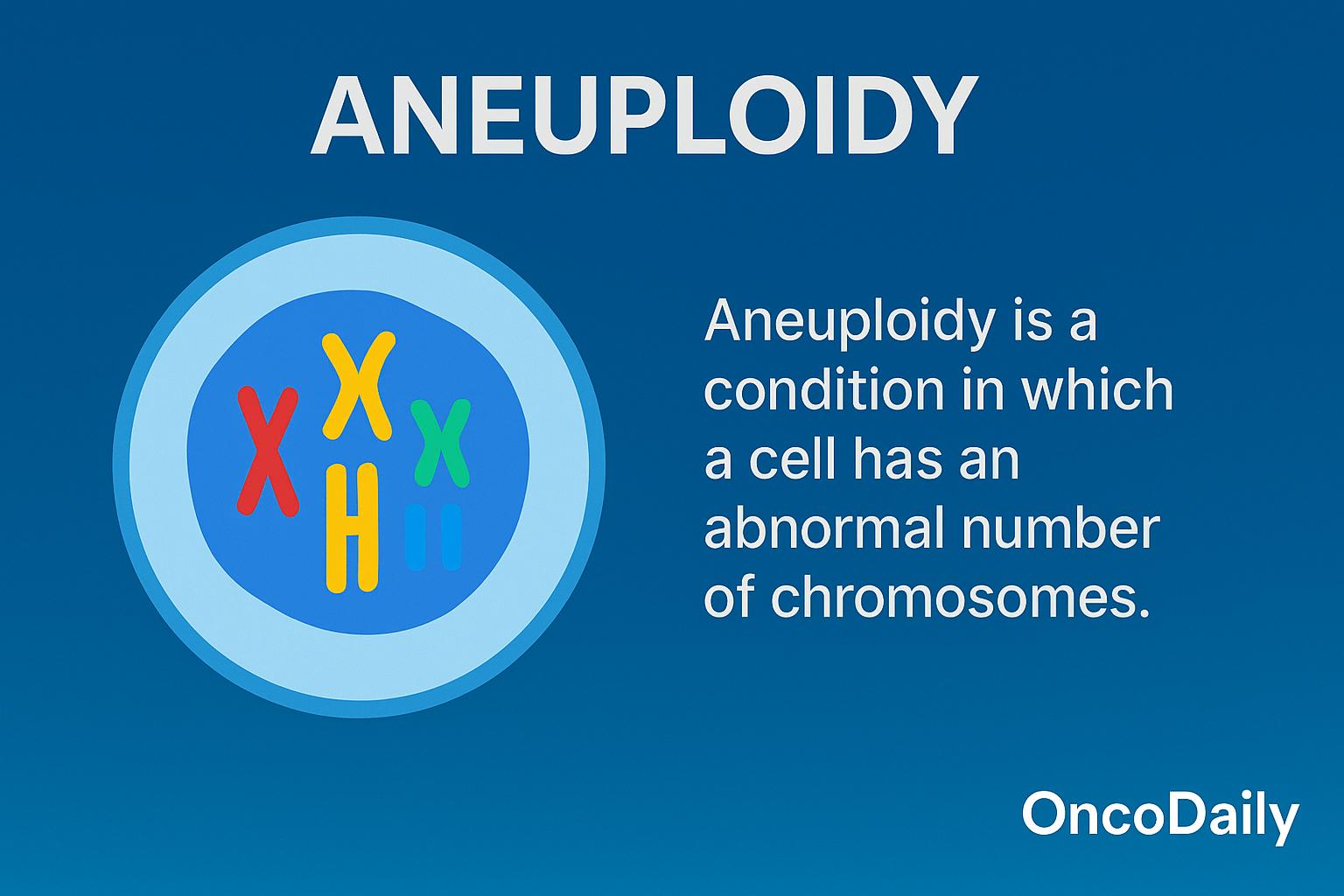
Aneuploidy refers to the presence of an abnormal number of chromosomes in a cell, deviating from the normal diploid set found in healthy human cells. Rather than having the standard 46 chromosomes, aneuploid cells may possess extra or missing chromosomes, resulting in chromosomal imbalances that disrupt normal gene expression and cellular homeostasis.
This condition is a hallmark of cancer biology and is observed in more than 90% of solid tumors and a substantial proportion of hematological malignancies. Once considered a passive consequence of genomic instability, aneuploidy is now recognized as an active contributor to cancer initiation and progression. It drives tumor evolution by promoting genetic diversity, enabling cancer cells to adapt to hostile environments and therapeutic pressure.
This article explores the multifaceted role of aneuploidy in cancer, examining its mechanistic origins, cellular consequences, contribution to tumor heterogeneity, and the emerging therapeutic strategies aimed at exploiting its vulnerabilities.
Mechanisms Leading to Aneuploidy in Cancer
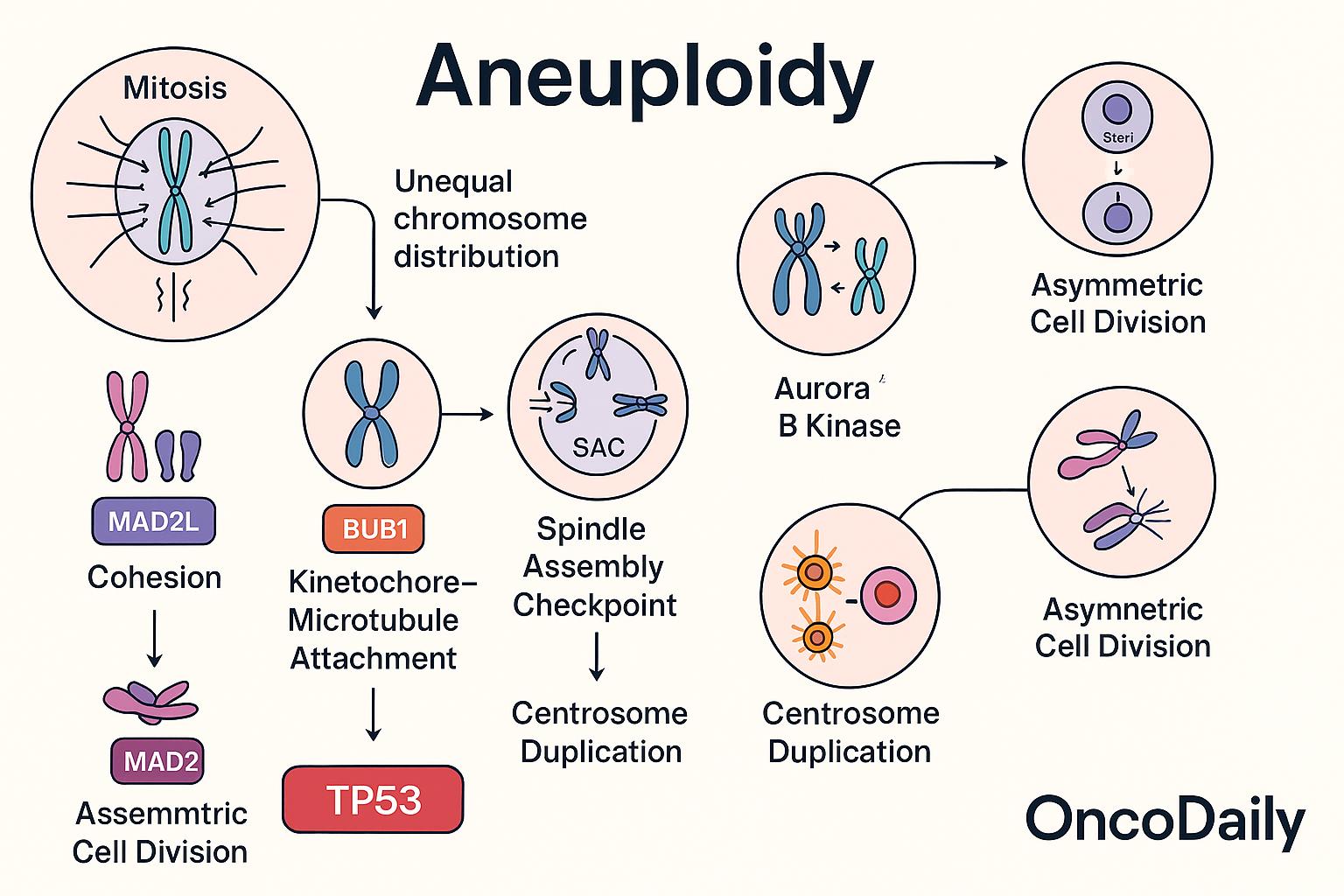
Aneuploidy primarily arises from errors in the complex and tightly regulated process of mitosis, during which replicated chromosomes are evenly segregated into daughter cells. Failures in several critical mitotic mechanisms can lead to unequal chromosome distribution, a phenomenon known as chromosomal missegregation.
One of the central safeguards against aneuploidy is the spindle assembly checkpoint (SAC), which ensures that all chromosomes are properly attached to the mitotic spindle before progression to anaphase. Deficiencies in SAC components—such as BUB1 (Budding Uninhibited by Benzimidazole 1) and MAD2L1 (Mitotic Arrest Deficient 2 Like 1)—compromise this checkpoint, allowing cells to proceed through mitosis with misaligned or unattached chromosomes. These proteins are often found to be mutated, downregulated, or epigenetically silenced in cancer, resulting in chromosome segregation errors and ongoing chromosomal instability.
Another critical process is centrosome duplication, which must occur once and only once per cell cycle to ensure proper bipolar spindle formation. Aberrant centrosome numbers can cause multipolar spindles, leading to unequal pulling forces and missegregation of chromosomes. Similarly, impaired chromosome cohesion—mediated by the cohesin complex—can lead to premature separation of sister chromatids, while defects in kinetochore-microtubule attachment can result in improper chromosome alignment and segregation. The Aurora B kinase (AURKB), a key regulator of chromosome-microtubule attachments and error correction during mitosis, is frequently dysregulated in cancers. Overexpression or malfunction of AURKB contributes to aneuploidy by failing to eliminate improper attachments, such as merotelic orientations, where one kinetochore is attached to microtubules from both spindle poles.
Asymmetric cell divisions, particularly in stem cells and early tumorigenic contexts, are another source of chromosomal instability. These divisions can exacerbate missegregation events and contribute to heterogeneous cell populations within tumors. Crucially, the TP53 tumor suppressor gene—often referred to as the “guardian of the genome”—plays a pivotal role in detecting and responding to aneuploidy. Under normal conditions, TP53 activation in response to mitotic stress or genomic imbalance leads to cell cycle arrest or apoptosis. However, in many cancers, TP53 is mutated or inactivated, allowing aneuploid cells to evade these checkpoints and continue proliferating. This tolerance enables the persistence and evolution of chromosomally unstable clones, facilitating tumor progression and therapeutic resistance.
Together, the failure of these interconnected cellular processes—especially when compounded by the loss of TP53 function—creates a permissive environment for aneuploidy, seeding genomic diversity and instability that underlie malignant transformation.
Functional Consequences of Aneuploidy in Tumor Cells
Aneuploidy disrupts the normal gene dosage balance in cells, creating profound cellular stress that, under typical circumstances, impairs cell fitness. The presence of extra or missing chromosomes leads to gene expression imbalances, affecting hundreds to thousands of genes simultaneously. This imbalance results in protein stoichiometry disturbances, where excess or deficient levels of protein subunits disrupt the formation and function of multi-protein complexes. These disruptions can overwhelm the proteostasis network, leading to proteotoxic stress, increased protein aggregation, and activation of cellular stress responses such as the unfolded protein response (UPR) and autophagy.
In normal cells, these stresses often lead to growth arrest, senescence, or apoptosis. However, cancer cells have evolved mechanisms to tolerate, and in some cases exploit, aneuploidy for selective advantage. By co-opting stress response pathways and bypassing cell cycle checkpoints—particularly through TP53 inactivation—cancer cells can survive and proliferate despite the inherent instability of aneuploid genomes.
Moreover, specific aneuploid karyotypes can confer context-dependent advantages to tumor cells. For instance:
-
Trisomy 7 is one of the most recurrent chromosomal alterations observed in colorectal cancer, renal cell carcinoma, and other epithelial malignancies. It can lead to the overexpression of genes such as EGFR and MET, contributing to enhanced growth signaling and proliferation.
-
Monosomy 3 in uveal melanoma is strongly associated with poor prognosis, early metastasis, and aggressive disease course. Loss of chromosome 3 often entails the loss of the tumor suppressor gene BAP1, which is involved in chromatin remodeling and DNA repair. This deletion allows tumor cells to escape growth suppression and facilitates metastasis.
-
In lung adenocarcinoma, gains of chromosomes 1q and 8q, harboring oncogenes such as MYC, can support increased proliferative capacity and resistance to apoptosis.
Aneuploidy also contributes to drug resistance by allowing tumor cells to generate genetic heterogeneity. This diversity increases the probability of pre-existing clones with resistance-conferring alterations, particularly under the selective pressure of chemotherapy or targeted agents. For example, aneuploid clones with extra copies of drug efflux transporters or downregulation of apoptotic genes can survive treatments that kill euploid counterparts.
Ultimately, while aneuploidy is energetically costly and destabilizing, cancer cells can adapt to and harness these genomic imbalances, transforming a vulnerability into a weapon for evolutionary fitness, treatment evasion, and tumor progression.
Aneuploidy and Tumor Heterogeneity
Chromosomal instability (CIN)—the persistent missegregation of chromosomes during cell division—is a principal driver of aneuploidy and a hallmark of many human cancers. Unlike static aneuploidy, CIN generates ongoing karyotypic variability, resulting in a heterogeneous population of tumor cells with divergent chromosomal compositions. This intra-tumor heterogeneity provides a substrate for natural selection, enabling tumors to adapt rapidly to changing environmental pressures, including immune surveillance, nutrient limitation, and—most critically—anticancer therapies.
As cells within a tumor acquire different chromosomal gains and losses, they develop diverse phenotypes. Some subclones may exhibit growth advantages, while others might develop mechanisms of drug resistance or immune evasion. Under the selective pressure of chemotherapy, for example, cells with karyotypes conferring resistance to apoptosis, enhanced efflux of drugs, or upregulation of detoxifying pathways are preferentially retained and expanded. This clonal selection leads to the emergence of resistant subpopulations that drive tumor recurrence or metastasis.
One illustrative example is seen in triple-negative breast cancer (TNBC), where CIN is especially high. In this setting, exposure to neoadjuvant chemotherapy can induce a shift in the clonal composition, with newly dominant subclones displaying chromosomal alterations linked to chemoresistance, such as amplifications of MYC or deletions of pro-apoptotic regulators like TP53BP1. Similarly, in colorectal cancer, longitudinal sampling of tumors before and after treatment has revealed dynamic karyotypic remodeling, with resistant metastases harboring novel aneuploidies not present in the primary lesion—suggesting ongoing CIN-fueled adaptation.
Furthermore, studies using single-cell sequencing have demonstrated that tumors with high CIN often exhibit branched evolutionary patterns, where distinct subclones evolve in parallel. These branches can contain chromosomally unique populations, each potentially capable of seeding recurrence or metastasis.
Ultimately, CIN-driven aneuploidy acts as an engine of tumor evolution, continuously reshaping the genetic landscape of cancer. This allows for phenotypic plasticity, making cancers more robust against treatment, harder to eradicate, and more likely to relapse. Therefore, targeting CIN or its downstream consequences is an area of active research, with the goal of reducing tumor adaptability and improving long-term treatment outcomes.
Prognostic and Diagnostic Value of Aneuploidy
Accumulating evidence suggests that the degree of aneuploidy in tumors has significant prognostic implications across various cancer types. High levels of aneuploidy—often assessed through measures of chromosomal aberrations or DNA content—have been repeatedly linked with poor clinical outcomes, including shorter overall survival, increased recurrence rates, and treatment resistance.
In breast cancer, particularly in estrogen receptor–positive (ER+) subtypes, studies have shown that high aneuploidy burden correlates with more aggressive tumor behavior and diminished response to endocrine therapy. Similarly, in non-small cell lung cancer (NSCLC), increased chromosomal instability and ploidy complexity are associated with worse prognosis and metastatic potential. A large pan-cancer analysis by Taylor et al. in Cancer Cell (2018) demonstrated that aneuploidy scores, based on somatic copy number alterations across the genome, predicted overall survival in multiple cancers, including lung, breast, and pancreatic adenocarcinoma.
Traditional assessment of tumor ploidy has relied on flow cytometry and digital image analysis of nuclear DNA content. Flow cytometry quantifies DNA content from tumor cell suspensions, distinguishing diploid from aneuploid populations based on DNA index values. Digital image cytometry, in contrast, uses histological sections and computer-assisted analysis to measure integrated optical density, offering insights into both ploidy and nuclear morphology. These methods remain useful in both clinical and research settings, particularly when evaluating archival samples or conducting retrospective outcome studies.
More recently, next-generation sequencing (NGS) approaches have enabled precise quantification of aneuploidy at unprecedented resolution. By analyzing copy number variations (CNVs) at both the chromosome-arm and whole-chromosome levels, researchers can now calculate aneuploidy scores from tumor exomes or low-pass whole genome sequencing data. In a study by Davoli et al. published in Cell (2017), such sequencing-derived aneuploidy scores were found to strongly correlate with immune evasion and inferior response to immunotherapy across The Cancer Genome Atlas (TCGA) datasets.
These sequencing-based biomarkers are now being explored for clinical integration, particularly as tools for risk stratification, prognostication, and monitoring minimal residual disease. For example, emerging liquid biopsy techniques can detect circulating tumor DNA (ctDNA) with aneuploid features, offering a noninvasive window into tumor dynamics and early detection of relapse.
Therapeutic Vulnerabilities Associated with Aneuploidy
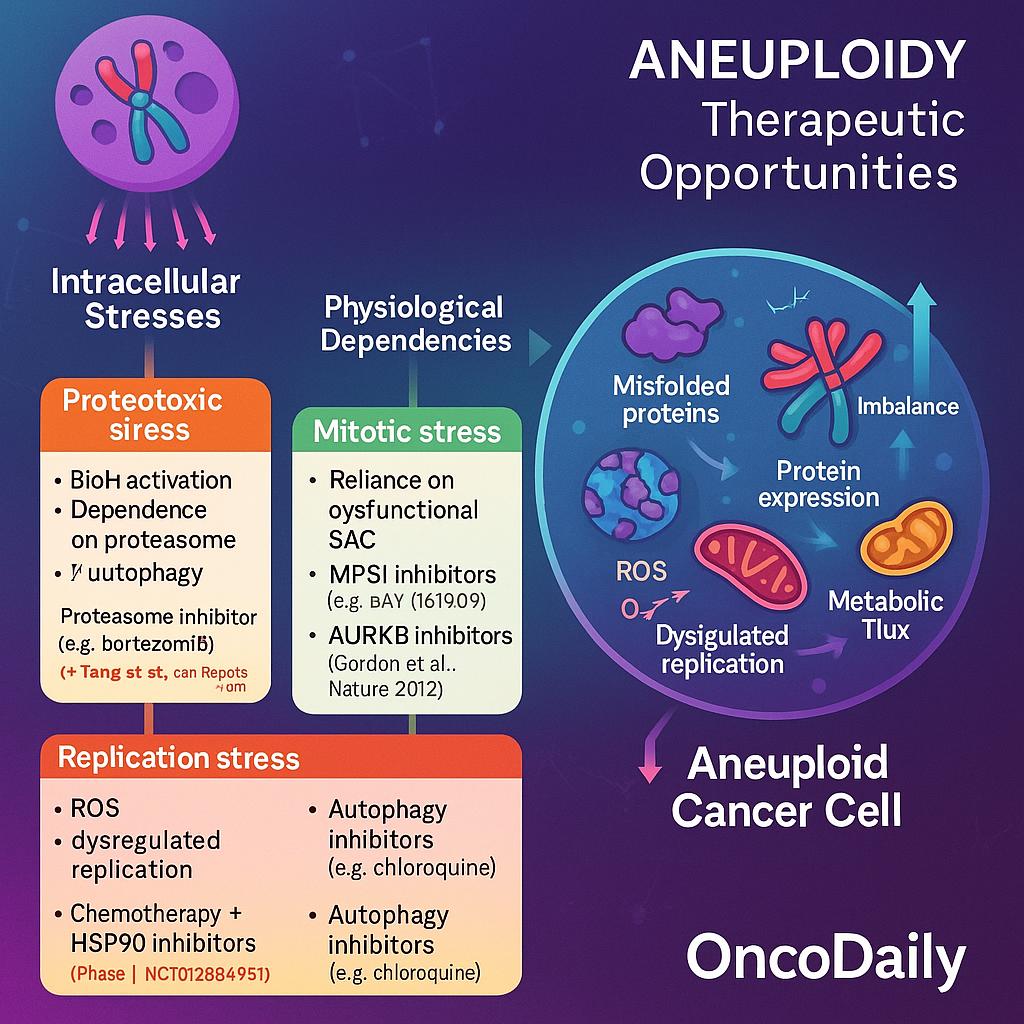
Aneuploid cancer cells experience profound intracellular stress due to imbalances in gene dosage, protein expression, and metabolic flux—pressures that normal diploid cells typically do not face. To cope with these disruptions, aneuploid cells develop distinct physiological dependencies, creating therapeutic vulnerabilities that can be selectively targeted.
One major consequence of aneuploidy is the accumulation of misfolded and excess proteins, a direct result of transcriptional and translational imbalance. This triggers activation of the unfolded protein response (UPR) and increases reliance on the proteasome and autophagy for protein quality control. Preclinical models have shown that aneuploid cells are more sensitive to proteotoxic stress, making them particularly susceptible to proteasome inhibitorslike bortezomib and HSP90 inhibitors such as geldanamycin derivatives. A study by Tang et al. (Cell Reports, 2011) found that human aneuploid cell lines were hypersensitive to HSP90 inhibition, likely due to impaired ability to buffer protein folding stress.
Another stress axis in aneuploid tumors is mitotic surveillance. These cells often harbor defects in the spindle assembly checkpoint (SAC) and rely heavily on its residual function to survive chaotic divisions. Kinases such as MPS1 (TTK)and AURKB (Aurora B), which regulate mitotic fidelity, have emerged as attractive targets. Inhibitors of MPS1, including BAY 1161909, have shown synthetic lethality in cells with high chromosomal instability. For instance, a study by Gordon et al. (Nature, 2012) demonstrated that SAC-deficient cells were selectively killed by low-dose MPS1 inhibition, underscoring this vulnerability.
Aneuploid tumors also exhibit increased oxidative stress and DNA replication stress, due to metabolic imbalance and impaired chromosome segregation. These features create opportunities for combination strategies that exploit stress overload. One such approach involves pairing DNA-damaging agents (e.g., platinum compounds, topoisomerase inhibitors) with agents that block adaptive stress pathways. For example, HSP90 inhibitors, which impair folding of key DNA repair proteins, can potentiate the cytotoxicity of chemotherapy in aneuploid settings. A phase I clinical trial (NCT01266451) explored ganetespib, an HSP90 inhibitor, in combination with chemotherapy in advanced solid tumors, showing early signs of efficacy in high CIN tumors.
Targeting autophagy is also under active investigation. Aneuploid cells often upregulate autophagy as a compensatory mechanism to clear damaged organelles and protein aggregates. Inhibitors like chloroquine or hydroxychloroquine—which block lysosomal acidification—may synergize with stress-inducing agents in aneuploid cancers. Although not yet standard, this strategy is under exploration in combination regimens.
Immunological Implications of Aneuploidy
Aneuploidy plays a paradoxical role in shaping the tumor–immune interface, acting both as a shield that facilitates immune evasion and as a trigger for innate immune activation. This dual behavior is tightly linked to the underlying genomic instability and cellular stress associated with abnormal chromosomal content.
On the immunosuppressive side, aneuploid tumors often exhibit downregulation of antigen processing and presentation machinery, including reduced expression of MHC class I molecules. This impairs cytotoxic T cell recognition and facilitates immune escape. Studies have shown that high levels of aneuploidy correlate with a “cold” immune microenvironment, characterized by low CD8+ T cell infiltration and upregulation of immunosuppressive cytokines such as TGF-β and IL-10. In a pan-cancer analysis by Davoli et al. (Science, 2017), tumors with greater somatic copy number alterations (SCNAs) exhibited lower expression of immune-related genes and worse responses to immune checkpoint blockade (ICB).
Conversely, chromosomal instability (CIN)—a driver of aneuploidy—can lead to micronuclei formation and leakage of chromosomal fragments into the cytosol, activating the cGAS-STING pathway. This innate immune sensor detects cytosolic DNA and induces type I interferon responses, potentially enhancing antitumor immunity and recruiting immune effector cells. However, this inflammatory signaling can be transient or dampened in tumors that evolve mechanisms to suppress STING signaling or adapt to chronic inflammation.
The net effect of aneuploidy on immunotherapy outcomes appears to depend on the balance between immune activation and suppression. In some contexts, high CIN may promote neoantigen diversity and immune visibility, but in others, it supports immune exclusion and resistance to ICB.
Efforts to use aneuploidy burden as a predictive biomarker are ongoing. Aneuploidy scores, derived from whole-exome or genome sequencing, have been evaluated in relation to immunotherapy efficacy. For instance, an analysis of The Cancer Genome Atlas (TCGA) data showed that high SCNA burden was associated with inferior survival following ICB, independent of tumor mutation burden (TMB). Still, these findings are tumor type–dependent and require prospective validation.
Emerging Technologies for Aneuploidy Detection
Aneuploidy detection in cancer has advanced with modern genomic tools that enable precise and often noninvasive assessment of chromosomal abnormalities. Whole-genome sequencing (WGS) offers comprehensive detection of copy number alterations across the genome but remains resource-intensive. In contrast, low-pass WGS provides a cost-effective option to quantify large-scale aneuploidy, especially useful in solid tumors.
Single-cell genomics allows analysis of individual cancer cells, revealing karyotypic diversity and uncovering rare resistant subclones—key in understanding tumor heterogeneity. Liquid biopsy, through analysis of circulating tumor DNA (ctDNA), is emerging as a noninvasive method to monitor aneuploidy in real time. Shallow WGS of cfDNA can detect chromosomal gains or losses and is already being tested in large studies like TRACERx and CIRCULATE-Japan.
Together, these tools not only improve diagnosis and monitoring but also support personalized treatment strategies by tracking chromosomal instability over time. As costs decrease, integration into routine oncology care is becoming increasingly feasible.
Future Directions and Research Challenges
Despite significant progress, key questions remain in aneuploidy research. One of the most critical is: what dictates whether aneuploidy is detrimental or beneficial to a cancer cell? While many aneuploid karyotypes induce cellular stress and impair viability, some confer strong growth or survival advantages—particularly under therapeutic pressure. Understanding this balance is central to designing effective interventions.
Another challenge is how to selectively exploit aneuploidy-related vulnerabilities—such as proteotoxic stress or metabolic imbalance—without damaging healthy, euploid cells. Therapeutic windows remain narrow, and toxicity concerns limit clinical translation of many promising compounds.
Looking forward, the integration of single-cell multi-omics, machine learning algorithms, and patient-derived organoids or xenografts offers a powerful toolkit to dissect the context-specific effects of aneuploidy. These approaches may reveal predictive markers of drug response, uncover novel therapeutic targets, and refine our understanding of how chromosomal imbalance shapes cancer evolution. Ultimately, a deeper mechanistic understanding will be essential for translating aneuploidy from a cytogenetic hallmark into a precision oncology target.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD