The ESMO Congress 2025 is a major global oncology event organized by the European Society for Medical Oncology (ESMO).
It is taking place at Messe Berlin in Berlin, Germany, from October 17 to 21, 2025. The congress features a comprehensive scientific and educational program designed to foster exchange and debate in translational cancer science, showcasing potentially practice-changing data, and stimulating multidisciplinary discussions to improve cancer treatment options.
Arndt Vogel, Head of the Visceral Oncology Center, Head of the Center for Personalized Medicine, MHH, and Senior Consultant of the Department of Gastroenterology, Hepatology and Endocrinology at Hannover Medical School, shared GI highlights from ESMO 2025 on X:
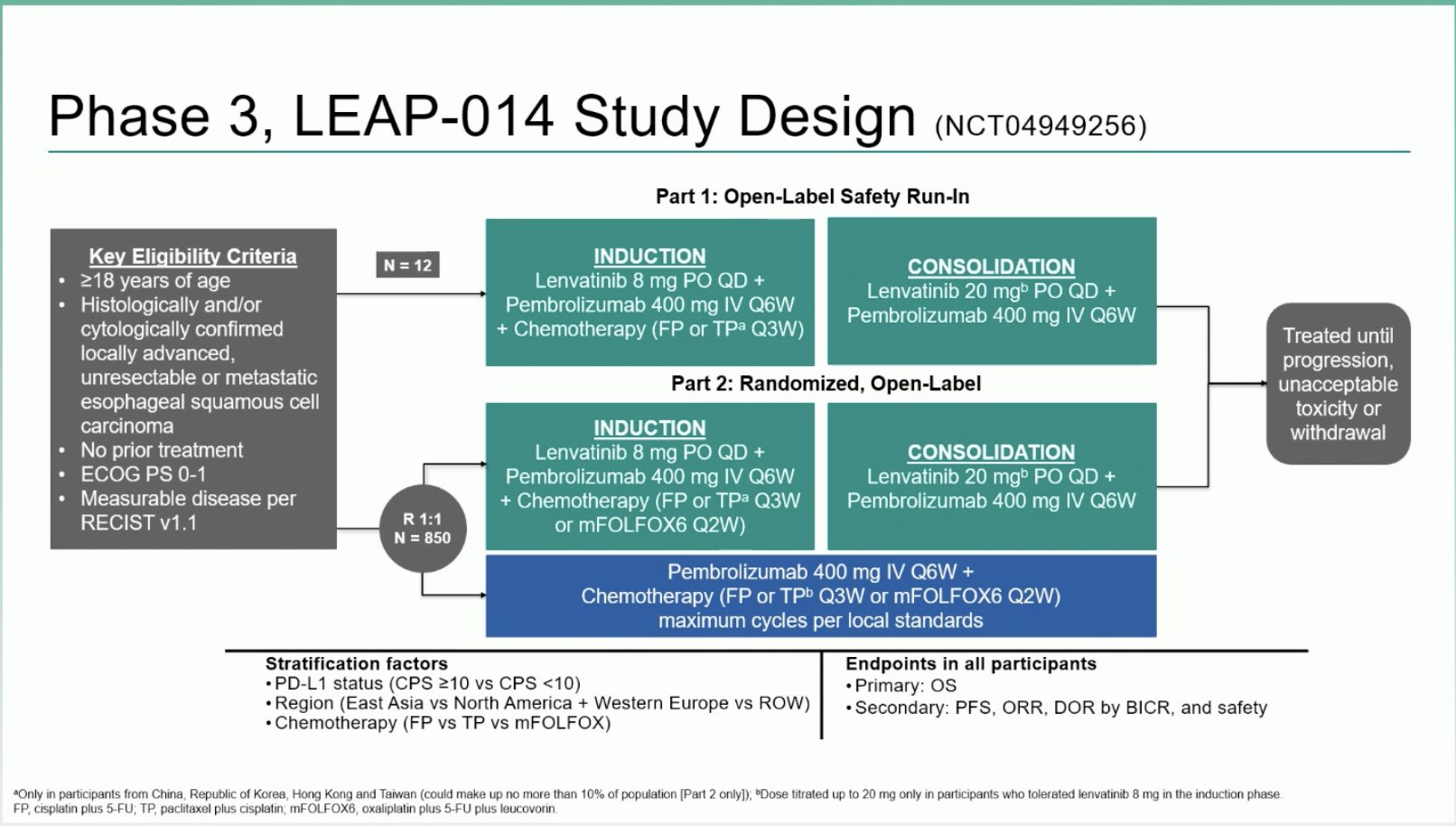
“LEAP-014: Lenvatinib plus CTx and pembrolizumab vs pembrolizumab plus CTx in metastatic ESCC
- ORR: 62 vs 54%
- mPFS: 7.2 vs 6.9 mo
- mOS: 17.6 vs 15.5 mo
- negative study, no benefit by addition of lenvatinib to CTx + Pembro, more toxicity.”
“Invited Discussant LBA81 and 2094O
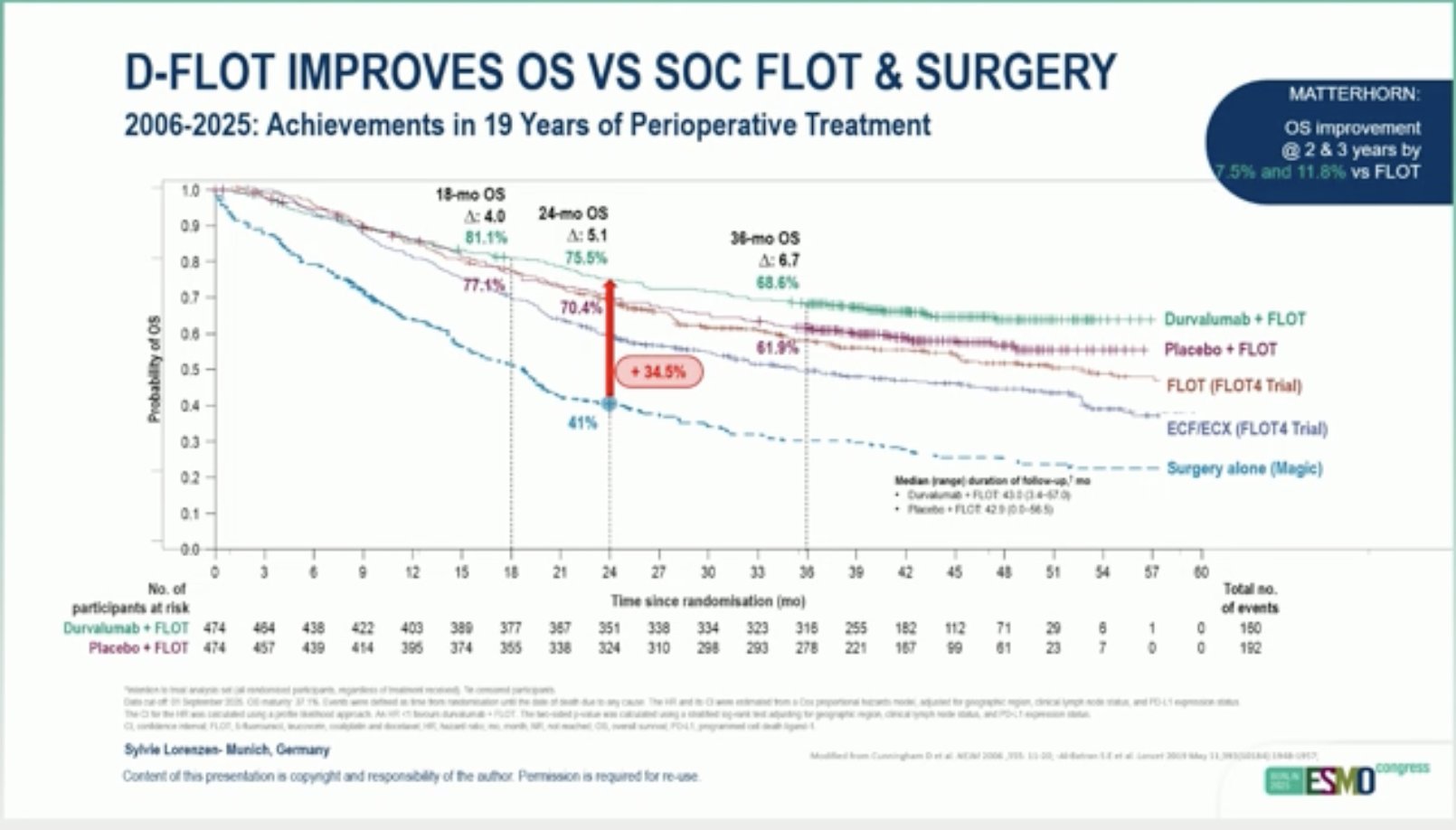
Durvalumab improves pCT and MPR in resectable G/ GEJ adenocarcinoma, with significant clinical benefit.”
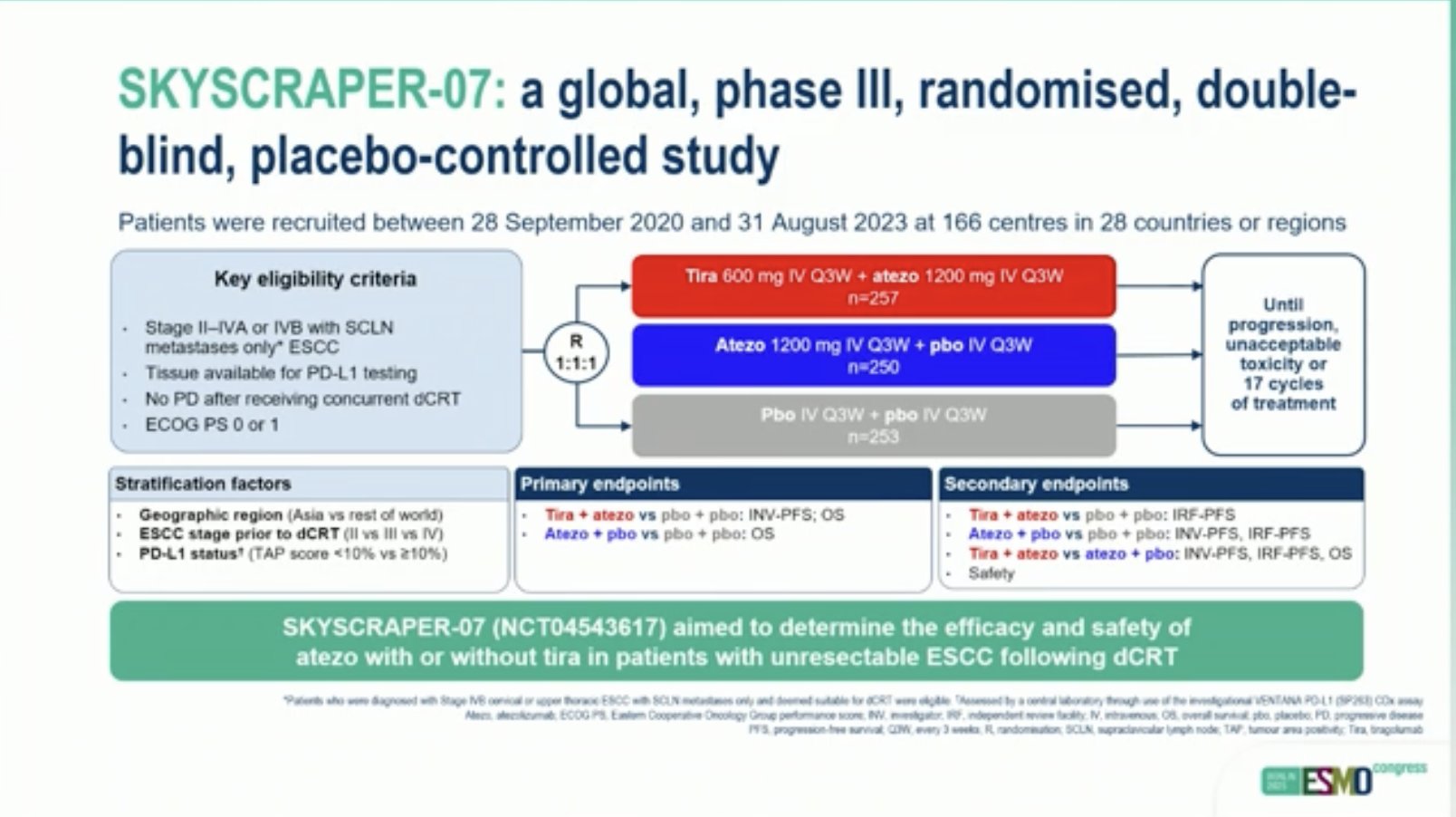
“SKYSCRAPER-07: Phase III study of atezo with or without tiragolumab in unresectable ESCC that has not progressed following dCRT
- mPFS: 29 vs 20.8 vs 16.6
- mOS: n.r. vs 38 vs 36 mo
- Atezo improves outcome, but adding tiragolumab to atezo with detrimental effect.”
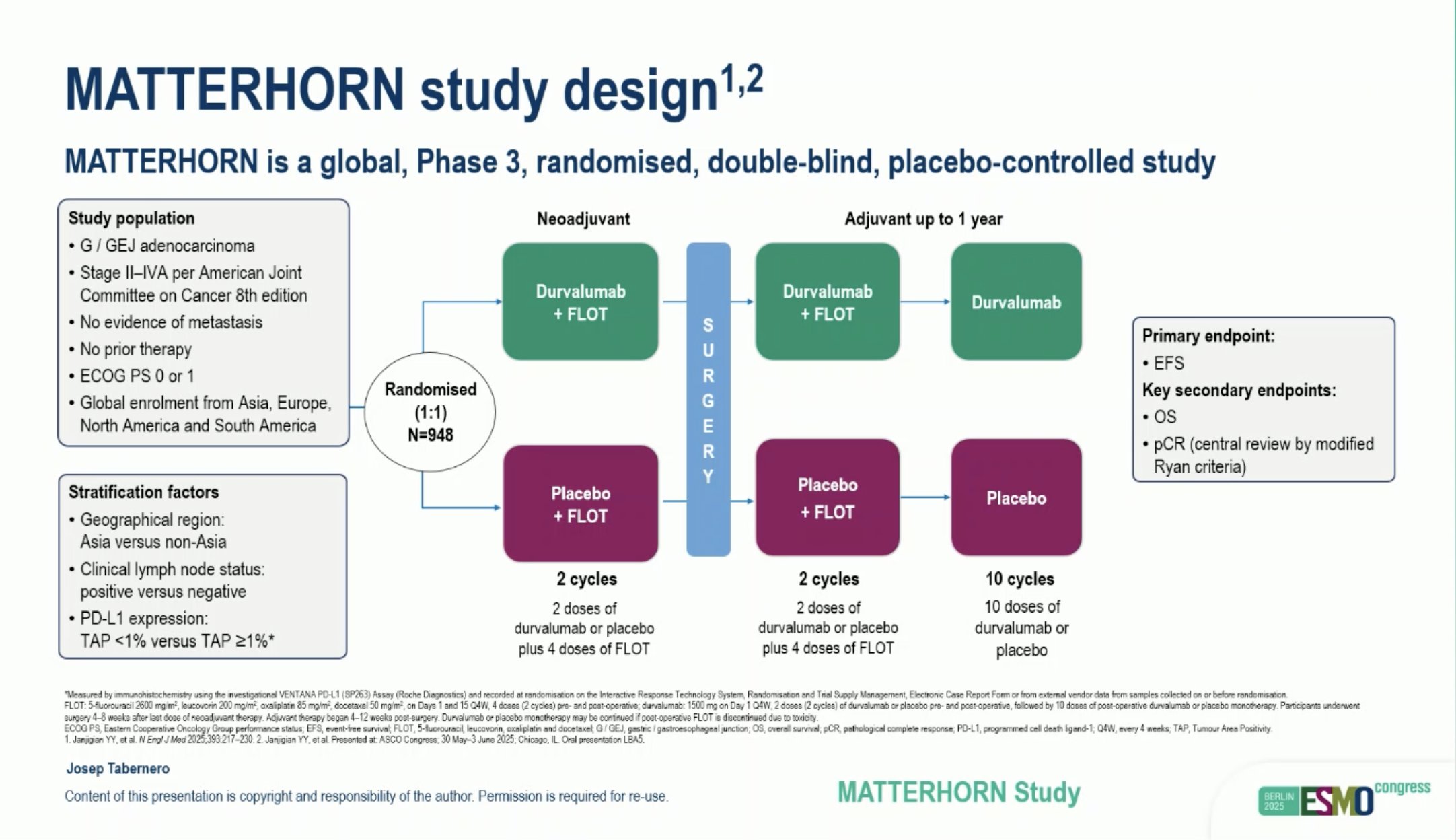
“MATTERHORN: Phase III study of durvalumab + FLOT in resectable G / GEJ adenocarcinoma
- pCR: 16%, MPR: 26%, any: 87%
- OS: HR: 0.78; 36-mo OS: 68 vs 61%
- OS improved independent of TAP, better in responders > new SOC.”
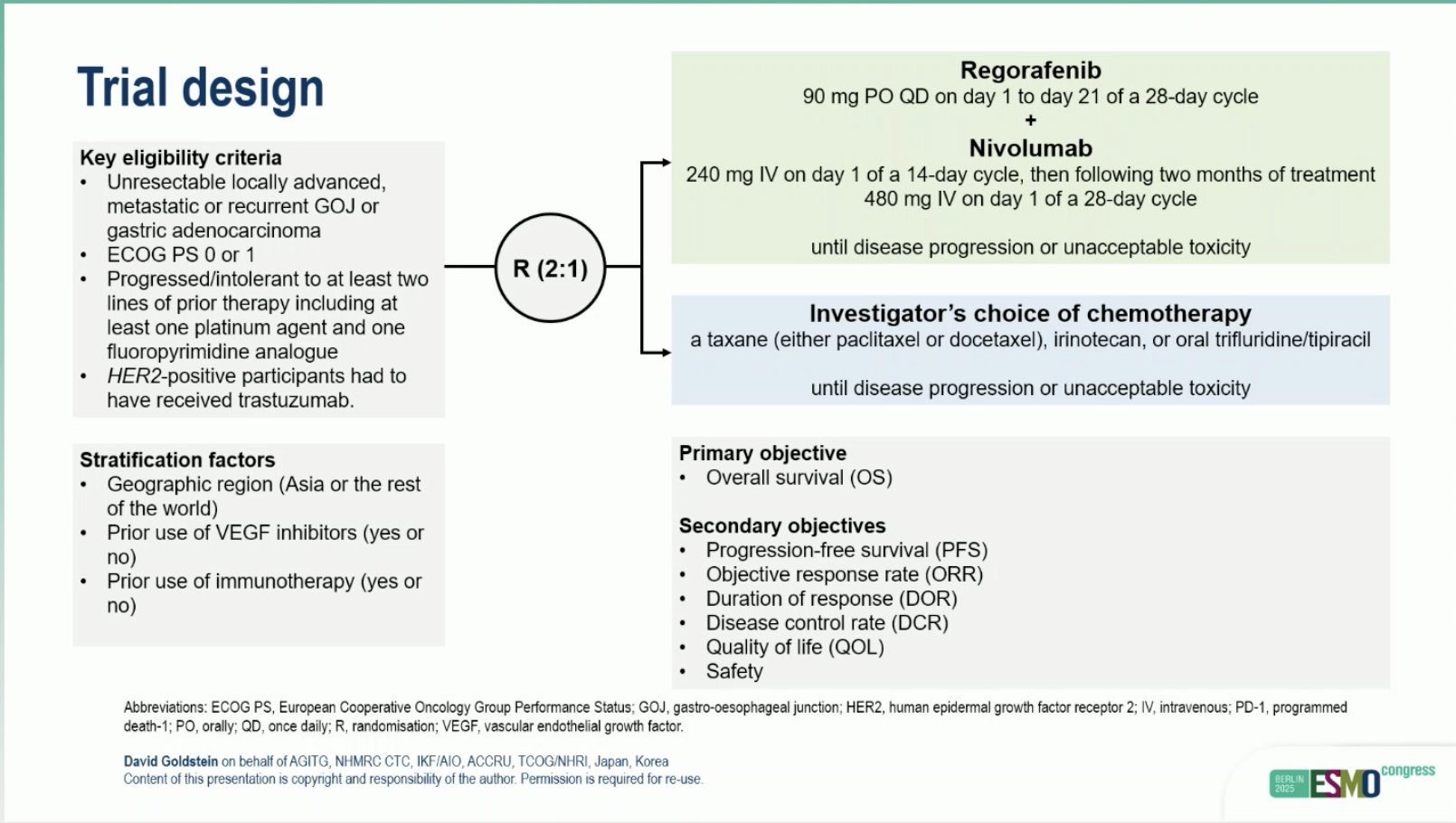
“INTEGRATE IIb: Regorafenib plus nivolumab vs investigator’s choice of CTx in previously treated G/ GEJ adenocarcinoma
- ORR: 7.4 vs 2.6%
- mPFS: 1.9 vs 1.9 mo
- mOS: 7.2 vs 6.9 mo
- study, but Rego may remain a backbone for chemo-free combinations.”
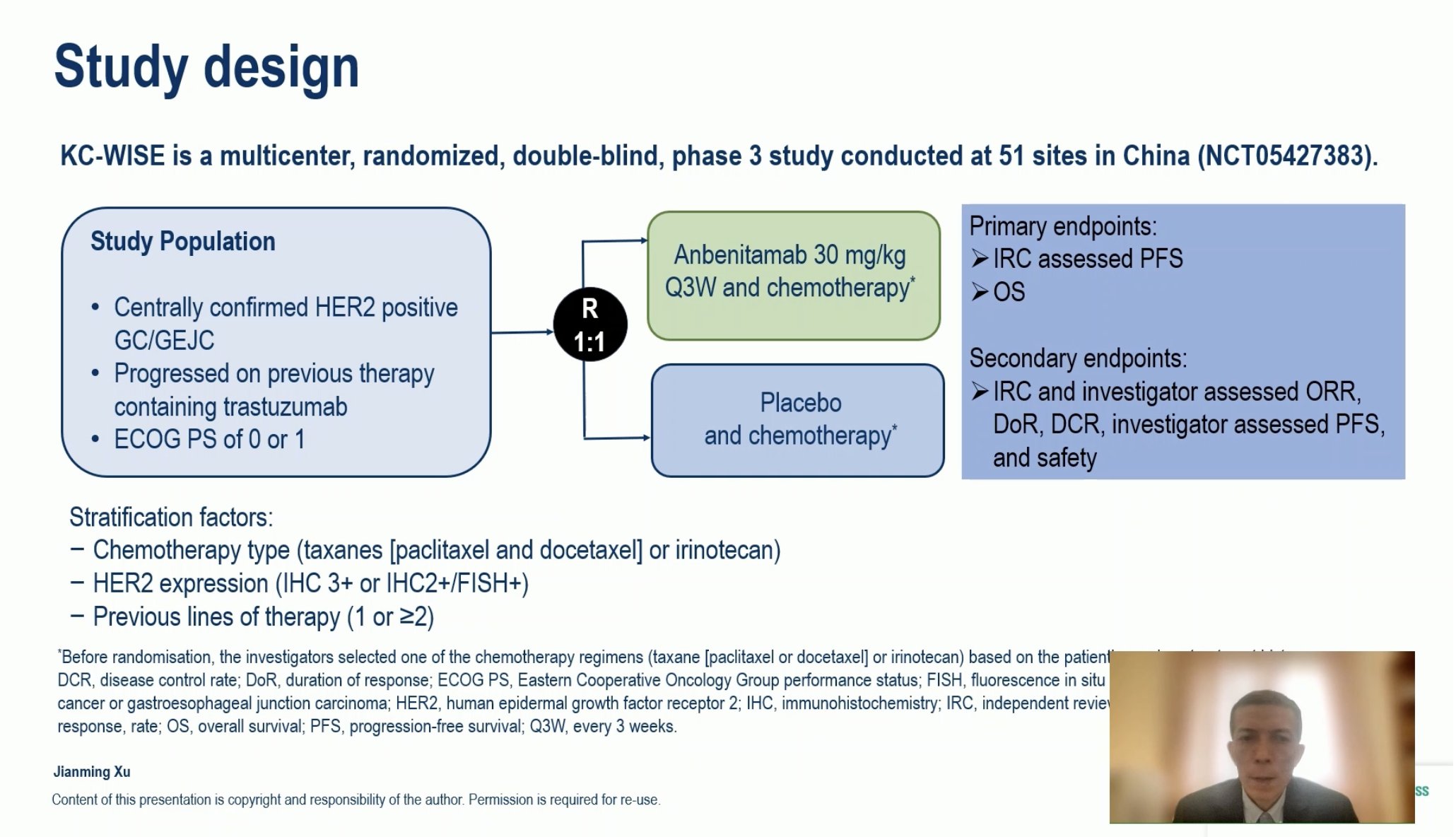
“KC-WISE: Anbenitamab plus chemotherapy for HER2-positive GC/GEJC after Trastuzumab treatment
- ORR: 55 vs 10.8%
- mPFS: 7.1 vs 2.7 mo
- mOS: 19.6 vs 11.5 mo
- Bi-specific antibody with significant benefit in HER2 pretreated patients.”
Follow the latest ESMO 2025 news on OncoDaily.
