TRYBECA-1 trial investigated eryaspase, a red blood cell–encapsulated asparaginase, in combination with standard second-line chemotherapy for advanced pancreatic ductal adenocarcinoma (PDAC). Given PDAC’s poor prognosis, with a global 5-year survival rate under 10% and limited efficacy of existing second-line options, the study aimed to determine whether targeting tumor metabolic dependencies through sustained asparagine depletion could improve patient outcomes.
Title: TRYBECA-1: A Randomized Phase III Study of Eryaspase Combined With Chemotherapy Versus Chemotherapy as Second-Line Treatment in Patients With Advanced Pancreatic Adenocarcinoma
Authors: Pascal Hammel, MD, Jean-Philippe Metges, MD , Teresa Macarulla Mercade, MD, Rocio Garcia-Carbonero, MD, PhD, Olivier Bouché, MD, Fabienne Portales, MD, Roberto A. Pazo Cid, MD, Laurent Mineur, MD, Antonio Cubillo Gracian, MD, Isabelle Trouilloud, MD, Rosine Guimbaud, MD, David Tougeron, MD, Juan Jose Reina, MD, Jaime Feliu, MD, Tamara Sauri, MD 0, Christos Fountzilas, MD, Thierry Lecomte, MD, Yann Molin, MD, Mariano Ponz-Sarvise, MD, Frederic Forget, MD, Rossana Berardi, MD, Eric Van Cutsem, MD, PhD, Fabio Gelsomino, MD, Christophe Tournigand, MD, Bruno Bockorny, MD, Jean Baptiste Bachet, MD, Miguel Marin Vera, MD, Pieter-Jan Cuyle, MD, Harpreet Wasan, MD, PhD, Marcus Noel, MD, Jean-Luc Van Laethem, MD, Richard Kay, PhD, Hagop Youssoufian, MD, Iman El-Hariry, MD, PhD, and Manuel Hidalgo, MD
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal cancers, with a global 5-year survival rate near 10%. After progression on first-line (1L) regimens such as FOLFIRINOX, NALIRIFOX, or gemcitabine/nab-paclitaxel, only about half of patients are fit enough for second-line (2L) therapy. Metabolic rewiring—driven largely by ubiquitous KRAS mutations—creates dependencies on amino acids such as asparagine (ASN) and glutamine (GLN).
Eryaspase, an asparaginase encapsulated within allogeneic red blood cells, was designed to sustain systemic ASN depletion while reducing classic asparaginase toxicities. A prior randomized phase IIb study (GRASPANC 2013-03) suggested an overall survival (OS) signal when eryaspase was combined with chemotherapy. TRYBECA-1, a large phase III trial, tested whether adding eryaspase to standard 2L chemotherapy improves outcomes in advanced PDAC.
Methods
Population. Adults (≥18 years) with histologically confirmed, unresectable stage III/IV PDAC and RECIST v1.1–measurable disease after progression on 1L therapy; ECOG 0–1 required. Key exclusions included >1 prior systemic regimen in the advanced setting, prior exposure or hypersensitivity to asparaginase, and inadequate organ function.
Patients were randomly assigned to 2 groups:
- eryaspase plus chemotherapy or
- chemotherapy alone.
Chemotherapy backbone was investigator’s choice based on prior treatment: gemcitabine (1,000 mg/m²) + nab-paclitaxel (125 mg/m²) on days 1, 8, 15 of 28-day cycles; or irinotecan-based therapy on days 1 and 15 (FOLFIRI: irinotecan 180 mg/m² + 5-FU 2,400 mg/m² 46-hour infusion with 400 mg/m² bolus + leucovorin 400 mg/m²; or nanoliposomal irinotecan 70 mg/m² + 5-FU/LV). Eryaspase was dosed at 100 U/kg IV on days 1 and 15 of each 28-day cycle. Treatment continued until progression, toxicity, or withdrawal.
The primary endpoint was OS in the intent-to-treat (ITT) population.Key secondary endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), duration of response (DoR), safety, and patient-reported outcomes (EORTC QLQ-C30).
Tumor assessments followed RECIST v1.1 by investigators and independent radiology review every 8 weeks. Pharmacokinetics/pharmacodynamics (PK/PD) assessed asparaginase activity and plasma ASN/GLN.
Study Design
TRYBECA-1 was a multicenter, international, randomized, open-label phase III trial (NCT03665441). Randomization (1:1) was stratified by ECOG (0 vs 1), chemotherapy regimen (gemcitabine/nab-paclitaxel vs irinotecan-based), and time from advanced diagnosis to randomization (<6 vs ≥6 months). The study targeted 390 deaths to provide ~88% power to detect HR 0.725 (one-sided α 2.5%) for OS, with an O’Brien–Fleming interim analysis.
Results
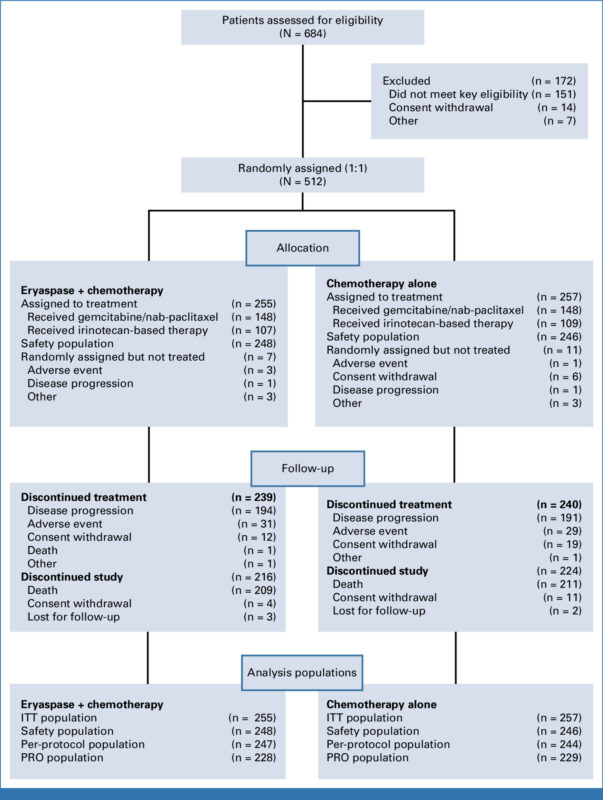
Of 684 screened, 512 were randomized between September 21, 2018 and December 14, 2020 to receive eryaspase + chemotherapy (n=255) or chemotherapy alone (n=257). Eighteen randomized patients discontinued before receiving treatment. Baseline features were well balanced.
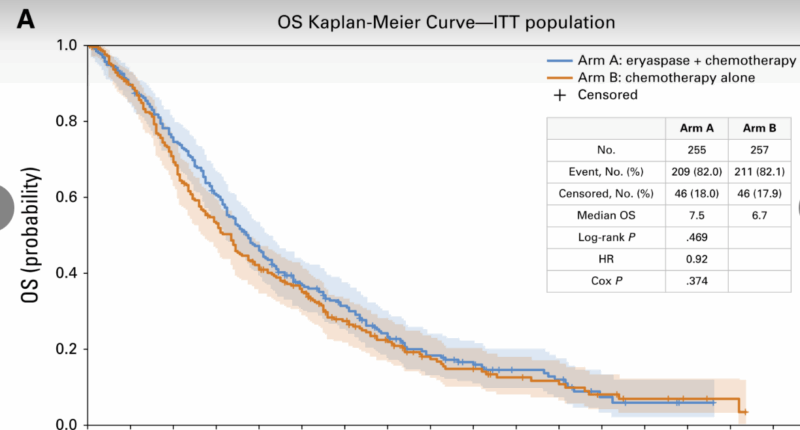
- Median OS was 7.5 months (95% CI, 6.5–8.3) with eryaspase + chemotherapy versus 6.7 months (95% CI, 5.4–7.5) with chemotherapy alone; HR 0.92 (95% CI, 0.76–1.11); P=.374. The primary endpoint was not met. Prespecified subgroup analyses showed no significant heterogeneity. In the irinotecan-based subgroup, a non-significant trend favored eryaspase (median 8.0 vs 5.7 months; HR 0.81; 95% CI, 0.60–1.09).
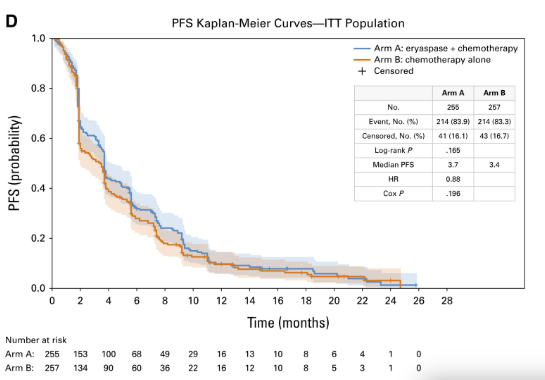
- Progression-Free Survival: Investigator-assessed median PFS was 3.7 months (95% CI, 3.4–4.1) vs 3.4 months (95% CI, 2.0–3.7); HR 0.88 (95% CI, 0.73–1.07); P=.196. Independent review was concordant.
- ORR was 16.1% vs 12.5% (odds ratio 1.35; 95% CI, 0.81–2.24; P=.242). DCR was numerically higher with eryaspase (57.6% vs 49.0%; P=.047), but duration of response was similar (median ~5.7–5.8 months).
- Mean global EORTC QLQ-C30 scores were broadly similar between arms over time.
Median trough asparaginase activity exceeded 500 U/L—well above the 100 U/L threshold regarded as pharmacologically active. Plasma ASN fell sharply after eryaspase administration, remaining well below baseline; GLN levels were largely unchanged. Exploratory analyses suggested that deeper ASN depletion (nadir < study-wide median of 15 µmol/L) might be associated with better OS within the eryaspase/irinotecan/5-FU subgroup (HR ~0.58), whereas GLN nadir did not stratify outcomes.
Neutralizing anti-asparaginase antibodies were detected post-baseline in 68.5% of eryaspase-treated patients, though trough activity generally remained above the pharmacologic threshold.
Safety
Nearly all patients experienced at least one treatment-emergent adverse event (TEAE). Grade ≥3 TEAEs were somewhat more frequent with eryaspase. Notable grade ≥3 events were neutropenia (25.4% vs 20.3%), asthenia (16.9% vs 13.8%), and anemia (17.3% vs 12.2%). TEAE-related discontinuations occurred in 18.5% vs 16.7%. TEAEs with fatal outcomes occurred in 5.6% vs 3.7% (study drug–related in 1.6% vs 0.8%). The safety profile was otherwise consistent with expectations for the respective chemotherapy backbones, with higher cumulative toxicity observed in the gemcitabine/nab-paclitaxel setting—likely reflecting prior FOLFIRINOX exposure in many patients.
Key Findings
- The primary endpoint was negative: adding eryaspase to standard 2L chemotherapy did not significantly improve OS (7.5 vs 6.7 months; HR 0.92; P=.374).
- PFS (3.7 vs 3.4 months; HR 0.88; P=.196) and ORR (16.1% vs 12.5%) showed no statistically significant benefit.
- DCR was numerically higher with eryaspase (57.6% vs 49.0%; P=.047), without longer duration of response.
- Pharmacodynamic target engagement (robust ASN depletion; trough activity >500 U/L) was achieved, but did not translate into survival benefit in the overall population.
- Safety was generally aligned with chemotherapy expectations, with modestly higher grade ≥3 hematologic and constitutional AEs in the eryaspase arm.\
- The trial provides large, contemporary benchmarks for gemcitabine/nab-paclitaxel and irinotecan-based regimens in the 2L PDAC setting.
Conclusion
In this international, randomized, phase III study of 512 patients with previously treated advanced PDAC, the addition of eryaspase to investigator’s choice 2L chemotherapy failed to improve overall survival, progression-free survival, or objective response rate versus chemotherapy alone, and produced a slightly higher rate of grade ≥3 hematologic and constitutional toxicities.
Robust pharmacologic activity (high trough asparaginase levels and marked ASN depletion) did not translate into clinical benefit in the overall population. TRYBECA-1 therefore does not support eryaspase as part of routine 2L management in unselected PDAC. The trial’s size and contemporary control outcomes provide useful benchmarks for future studies and underscore the need to pivot toward biomarker-driven and mechanism-based combinations that target PDAC’s fundamental oncogenic and microenvironmental biolog
You can read the full article here.