
New Paper Alert: Hepatocellular Carcinoma – Stereotactic Body Radiotherapy vs Sorafenib Alone in the NRG Oncology/RTOG 1112 Phase 3 Randomized Clinical Trial – JAMA Oncology
In the latest study published in JAMA Oncology on December 19, 2024, researchers led by Laura A. Dawson, MD, explore the efficacy of combining stereotactic body radiation therapy (SBRT) with Sorafenib versus using Sorafenib alone in treating locally advanced hepatocellular carcinoma (HCC). This Phase 3 randomized clinical trial, known as NRG Oncology/RTOG 1112, addresses a critical gap in treatment options for HCC patients, particularly those with macrovascular invasion who are not candidates for standard therapies.
Authors: Laura A. Dawson, MD; Kathryn A. Winter, MS; Jennifer J. Knox, MD; Andrew X. Zhu, MD, PhD; Sunil Krishnan, MD; Chandan Guha, MD; Lisa A. Kachnic, MD; Michael T. Gillin, PhD; Theodore S. Hong, MD; Timothy D. Craig, PhD; Terence M. Williams, MD, PhD; Ali Hosni, MBBCh; Eric Chen, MD; Anne M. Noonan, MBBCh; Eugene J. Koay, MD; Rishi Sinha, MD; Michael I. Lock, MD; Nitin Ohri, MD; Jennifer A. Dorth, MD; Guila Delouya, MD; Anand Swaminath, MD; Jennifer Moughan, MS; Christopher H. Crane, MD
Published in JAMA Oncology, December 19, 2024
Background
Hepatocellular carcinoma (HCC) continues to pose significant challenges globally, particularly in locally advanced stages where recurrence within the liver after systemic therapy is common. Although Sorafenib has been the standard first-line treatment, its efficacy in enhancing survival is not entirely satisfactory. This phase 3 trial explores whether adding stereotactic body radiation therapy (SBRT) to Sorafenib can improve survival and disease control in patients with locally advanced HCC, many of whom have macrovascular invasion (MVI) and are ineligible for other local therapies.
Methods
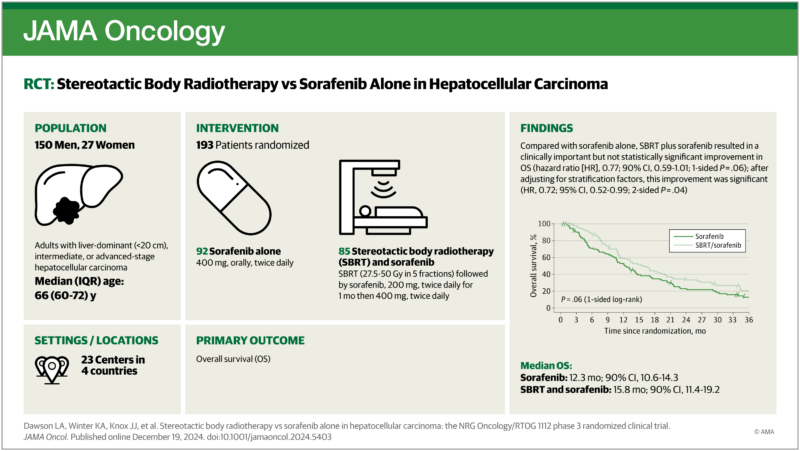
This multicenter phase 3 randomized clinical trial compared the effectiveness of Sorafenib alone versus Sorafenib combined with SBRT in patients with locally advanced HCC. The study involved patients unsuitable for or resistant to standard local-regional therapies. Treatment included personalized SBRT doses ranging from 27.5 to 50 Gy delivered in 5 fractions. Patient stratification was based on performance status, liver function, metastases presence, and macrovascular invasion.
Study Design
- Population: 193 patients randomized; 177 eligible for analysis. Median age: 66 years. 84.7% male.
- Interventions:
- Sorafenib monotherapy.
- SBRT (27.5-50 Gy in 5 fractions) followed by sorafenib.
- Primary Endpoint: Overall survival (OS).
- Secondary Endpoints: Progression-free survival (PFS), adverse events, and quality of life.
Results
- Overall Survival (OS):
- Median OS with sorafenib: 12.3 months (90% CI, 10.6–14.3).
- Median OS with SBRT + sorafenib: 15.8 months (90% CI, 11.4–19.2).
- Adjusted HR for OS: 0.72 (95% CI, 0.52–0.99; P = .04), favoring SBRT.
- Progression-Free Survival (PFS):
- Median PFS with sorafenib: 5.5 months (95% CI, 3.4–6.3).
- Median PFS with SBRT + sorafenib: 9.2 months (95% CI, 7.5–11.9).
- HR for PFS: 0.55 (95% CI, 0.40–0.75; P < .001).
- Adverse Events:
- Grade 3 or higher events: 42% (sorafenib) vs. 47% (SBRT + sorafenib).
- Treatment-related deaths: 2 (sorafenib group) vs. 1 (SBRT + sorafenib group).
- Quality of Life:
- Improved quality of life at 6 months: 10% (sorafenib) vs. 35% (SBRT + sorafenib).
Key Findings
- SBRT combined with sorafenib improved OS (median: 15.8 vs. 12.3 months P=.06)
- PFS significantly improved with SBRT and sorafenib (median: 9.2 vs. 5.5 months; P < .001).
- Adverse events were comparable between groups
- Quality of life improvements were more frequent in the SBRT + sorafenib group (35% vs. 10%).
Key Takeaway Messages
- SBRT combined with Sorafenib offers a potential clinical benefit in managing locally advanced HCC, particularly in improving progression-free survival and quality of life.
- These findings support the consideration of SBRT as a valuable adjunct to systemic therapy for patients with advanced HCC, especially those with large tumors and MVI.
Conclusion
This phase 3 trial demonstrates that SBRT combined with sorafenib improves progression-free survival and quality of life for patients with locally advanced HCC. While the observed overall survival benefit did not reach statistical significance, the findings underscore the potential of SBRT as an effective adjunct to systemic therapy, warranting further investigation in future trials.

You can Read the Full Article in Jama Oncology!
Summary by Sona Karamyan, MD




-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
