Piotr Wysocki recently posted on LinkedIn:
“For many years, anti-inflammatory drugs, mainly aspirin and celecoxib, have been studied for their activity as adjuvant treatment in patients with early colorectal cancer. Both aspirin and celecoxib inhibit cyclooxygenase (COX) enzymes, which are crucial in converting arachidonic acid to prostaglandins. Aspirin inhibits both COX-1 and COX-2, while celecoxib is a selective COX-2 inhibitor.
Data on 964 patients with rectal or colon cancer from the Nurses’ Health Study and the Health Professionals Follow-up Study patients with mutated-PIK3CA colorectal cancers, regular use of aspirin after diagnosis was associated with significantly superior cancer-specific survival (HR=0.18) and overall survival (HR=0.54). A phase III trial (PRODIGE 50-ASPIK) currently evaluates aspirin’s impact on disease-free survival (DFS) in stage III or high-risk stage II colon cancer patients with PIK3CA mutations.
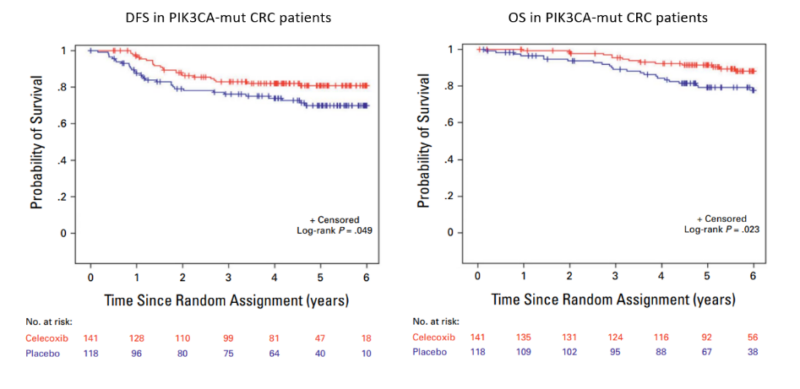
et al. published the results of a randomized phase III study (CALGB/SWOG 80702) that analyzed the impact of celecoxib in patients undergoing adjuvant chemotherapy. The study, conducted in a 2×2 fashion, evaluated the impact of the duration of FOLFOX chemotherapy (3 v. 6 months) and the use of celecoxib or placebo for 3 years after surgery. The study did not demonstrate significant improvement in disease-free survival (DFS) with celecoxib in the general population.
However, patients with PIK3CA activating mutations treated with celecoxib exhibited improved DFS (adjusted HR, 0.56 [95% CI, 0.33 to 0.96]) compared with PIK3CA wildtype patients. Overall survival (OS) was also significantly improved for patients with PIK3CA activating mutations treated with celecoxib (adjusted HR, 0.44 [95% CI, 0.22 to 0.85]). The results were especially striking (HR for OS=0.35 (95% CI, 0.16 to 0.77) when the analysis excluded PIK3CA-mutated patients taking aspirin.
Despite the lack of final confirmation from prospective clinical trials evaluating anti-inflammatory drugs in PIK3CA-mutated CRC patients, the available, robust, and consistent data are reassuring. They support a case-by-case discussion on the potential postoperative use of aspirin in patients without apparent contraindications to non-steroidal anti-inflammatory agents.”
Source: Piotr Wysocki/LinkedIn
Piotr Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy.
His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment.
Read other posts by Piotr Wysocki published on OncoDaily.
