AtezoTRIBE Study: Adding Atezolizumab to FOLFOXIRI/Bevacizumab Improves Survival in Metastatic Colorectal Cancer, Especially in Biomarker-Selected Patients
Authors: Carlotta Antoniotti, MD, PhD, Daniele Rossini, MD, PhD, Filippo Pietrantonio, MD, Lisa Salvatore, MD, PhD, Sara Lonardi, MD, Stefano Tamberi, MD, PhD, Federica Marmorino, MD, PhD, Roberto Moretto, MD, Michele Prisciandaro, MD, Emiliano Tamburini, MD, Giampaolo Tortora, MD, PhD, Alessandro Passardi, MD, Francesca Bergamo, MD, Alessandra Raimondi, MD, Giuliana Ritorto, MD, Beatrice Borelli, MD, PhD, Veronica Conca, MD, Clara Ugolini, MD, PhD, Giuseppe Aprile, MD, Lorenzo Antonuzzo, MD, Fabio Gelsomino, MD, Erika Martinelli, MD, PhD, Nicoletta Pella, MD, Gianluca Masi, MD, Luca Boni, MD, Jerome Galon, PhD, Chiara Cremolini, MD, PhD
Published in the Journal of Clinical Oncology on June 14, 2024
Design and Methods.
The AtezoTRIBE study (NCT03721653) was a prospective, open-label, randomized phase II trial conducted in 22 Italian centers. A total of 218 patients with unresectable, previously untreated metastatic colorectal cancer (mCRC) were randomized 1:2 to receive either FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) plus bevacizumab (control group, n=73) or FOLFOXIRI plus bevacizumab and the immune checkpoint inhibitor atezolizumab (experimental group, n=145).
The primary endpoint was progression-free survival (PFS), and key secondary endpoints included overall survival (OS), second PFS, safety, and objective response rate (ORR). Exploratory analyses investigated the predictive impact of mismatch repair (MMR) status, tumor mutational burden (TMB), and Immunoscore Immune-Checkpoint (IC) status.
Introduction
While immunotherapy has improved outcomes in the ~5% of mCRC patients with deficient MMR (dMMR) tumors, making this approach effective in proficient MMR (pMMR) tumors has proven challenging.
The AtezoTRIBE study hypothesized that adding the immune checkpoint inhibitor atezolizumab to first-line FOLFOXIRI plus bevacizumab could increase tumor immunogenicity and promote anti-tumor immune responses, thereby improving efficacy in the pMMR population.
What We Learned
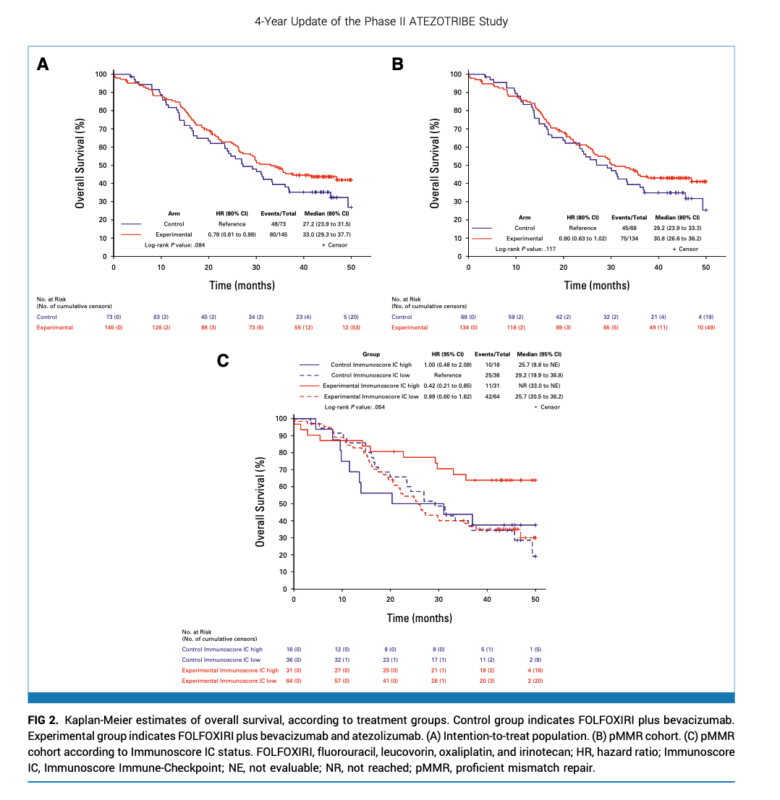
At a median follow-up of 45.2 months, 128 (59%) deaths and 177 (81%) PFS events occurred. In the intention-to-treat (ITT) population, adding atezolizumab significantly prolonged PFS (median 13.1 vs 11.5 months; HR 0.72, P=0.018) and demonstrated a strong trend for improved OS (median 33.0 vs 27.2 months; HR 0.78, P=0.084). In the pMMR cohort (n=202), median OS was 30.8 vs 29.2 months for experimental and control arms (HR 0.80, P=0.117). Updated ORR was similar between groups.
Key Highlights
- In the pMMR cohort, interactions were seen between treatment and TMB (P=0.043) and Immunoscore IC (P=0.092), with greater atezolizumab benefit in TMB-high (≥10 mutations/Mb) and Immunoscore IC-high (high PD-L1/CD8 proximity) subgroups.
- Median OS was not reached vs 29.2 months in TMB-high pMMR patients receiving atezolizumab vs control (HR 0.39).
- In Immunoscore IC-high pMMR, median OS was 41.7 vs 29.6 months with atezolizumab (HR 0.59).
- TMB and Immunoscore IC were independent predictors of OS/PFS benefit in multivariate analyses of the pMMR cohort.
- No new safety signals emerged with ~4 year follow-up.
Key Takeaways
- Adding atezolizumab to first-line FOLFOXIRI/bevacizumab improves outcomes in mCRC, with significant PFS benefit in the ITT population and promising OS trends, particularly in biomarker-selected pMMR subgroups.
- TMB and Immunoscore IC represent potential predictive biomarkers for identifying pMMR patients most likely to benefit from this ICI-based regimen.
- A phase III trial evaluating atezolizumab + FOLFOXIRI/bevacizumab specifically in pMMR, Immunoscore IC-high mCRC is planned based on these findings.
Summary by Amalya Sargsyan, MD


