Piotr Wysocki recently posted on LinkedIn:
“Results of a Destiny-Breast06 trial have been presented by Giuseppe Curigliano at the ASCO Annual Meeting.
The study enrolled 866 chemotherapy-naïve patients with HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (HER2 IHC=0 with membrane staining) metastatic breast cancer (mBC) patients who failed at least one line of endocrine treatment in the palliative setting. Patients were randomized (1:1) to the trastuzumab-deruxtecan (T-DXd) arm or the chemotherapy of physician choice (TPC) arm. Patients in the TPC arm could receive capecitabine, nab-paclitaxel, or paclitaxel at the physician’s discretion.
The primary endpoint of the study was PFS in HER2-low patients and secondary endpoints included PFS in the ITT population (HER2-low and HER2-ultralow), and OS in HER2-low and ITT populations.
The results were as follows:
- PFS in HER2-low – HR=0.62 95%CI 0.51-0.74 with median PFS of 13.2 months (T-DXd) and 8.1 months (TPC)
- PFS in ITT – HR=0.63 95%CI 0.53-0.75 with median PFS of 13.2 months (T-DXd) and 8.1 months (TPC)
- OS in HER2-low – HR=0.83 95%CI 0.66-1.05
- OS in ITT – HR=0.81 95%CI 0.65-1.00
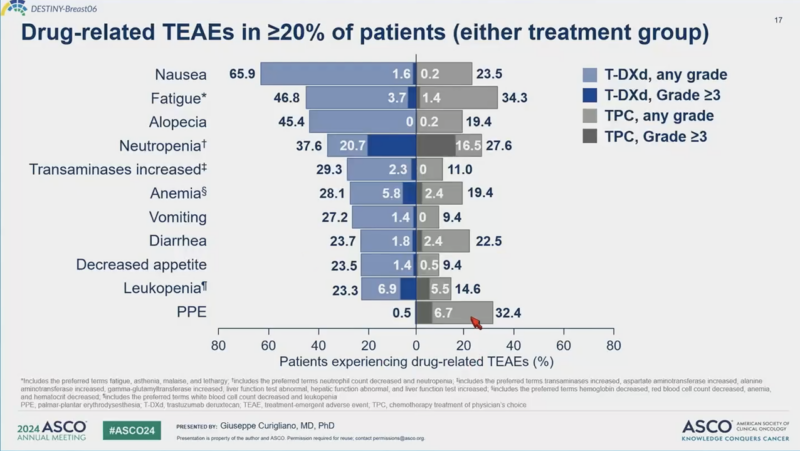
The significant improvement in PFS was achieved at the cost of the high toxicity associated with T-DXd, which may have significantly impeded patients’ quality of life.
The Destiny Breast06 study demonstrated that T-DXd is active in patients with HER2-low and HER2-ultralow mBC. However, a clinically relevant question is how many ER+ mBC patients require such an aggressive approach immediately after endocrine treatment failure.
I cannot imagine offering T-DXd to asymptomatic or mildly symptomatic patients at no imminent risk of visceral crisis (the majority of our ER+ mBC patients). Such patients care about the quality of their lives, and the treatment decisions must consider this fact. This patient population usually does not require fast/profound responses, and disease stabilization achieved at a low cost (toxicity) may represent the optimal goal.
To achieve such a goal, one can use well-known and inexpensive therapeutic options like sequential systemic treatment based on multidrug metronomic chemotherapy (e.g., VEX) or single agent-based intravenous weekly regimens instead of the toxic (biologically and financially) ADC.”
Authors: Giuseppe Curigliano, Xichun Hu, Rebecca Alexandra Dent, Kan Yonemori, Carlos H. Barrios, Joyce O’Shaughnessy, Hans Wildiers, Qingyuan Zhang, Seock-Ah Im, Cristina Saura, Laura Biganzoli, Joohyuk Sohn, Christelle Levy, William Jacot, Natasha Begbie, Jun Ke, Gargi Surendra Patel, Aditya Bardia
Source: Piotr Wysocki/LinkedIn
Piotr Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy.
His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment.
Read other posts by Piotr Wysocki published on OncoDaily.


