Percy Lee, Professor and Vice-Chair of Clinical Research at City of Hope, shared on X/Twitter:
”Hot off the press. HyCRT-SABR for unresectable locally advanced NSCLC in JAMA Oncology! Tweetorial of key findings and historical perspective.

This was a concept/trial conceived in 2016, protocol writing, enrollment, inexperience, professional moves, data analysis, writing all factored in how long this took (life lessons for me). Nevertheless, I am happy to report that it is finally online. Performed at UCLA Health but took a village/team to get the trial completed.
The protocol was conceived pre-PACIFIC, and pre-results of RTOG 0617. We assumed that dose escalation for stage III unresectable NSCLC will ‘for sure’ translate to better loco-regional control, and overall survival. We hypothesized, though, that using SABR techniques and approaches would translate to further gains while maintaining safety of treatment!
Even in PACIFIC intra thoracic disease progression still dominates as primary mode of reatment failure in these patients treated with CRT, followed by consolidative durvalumab out-back AstraZeneca.
No treatment breaks were allowed. Essentially all study patients completed CRT w/ platinum doublet w/in 3 weeks. Consolidation chemotherapy was given at the discretion of treating medical oncologist. Trial design was based on pre-specified stopping rules for 90-day acute treatment related-intra thoracic toxicities.
Patients advanced to higher SABR boost dose if dose-limiting toxicities (any >= grade 3 pulmonary, GI, or cardiac toxicities, or any non-heme >= grade 4) occurred in fewer than 1/3 of the patients w/in 90 days.
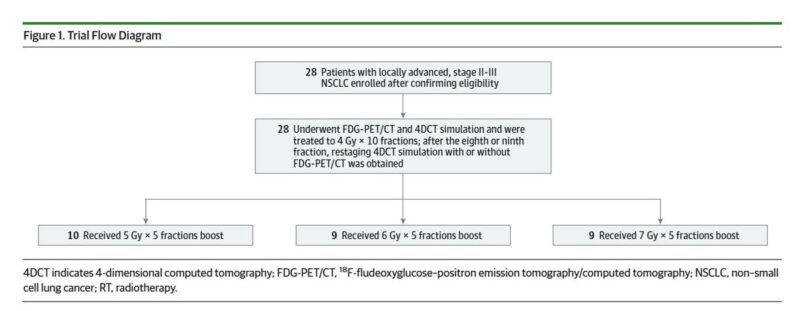
All patients received 4 Gy x 10 treatments followed an adaptive SABR boost to residual metabolically active disease consisting of an additional 25 Gy (low 5 Gy x 5), 30 Gy (intermediate, 6 Gy x 5), or 35 Gy (high, 7 Gy x 5) with chemo. Total doses: 65-75 Gy in 15 treatments.
Due to the era of enrollment, only one patient (last one) received consolidation durva. Primary outcome: Determine the maximally tolerated radiation dose! Enrollment: May 2011 to May 2018. Median f/u was 18.2 months. 28 patients enrolled, 86% stage III.
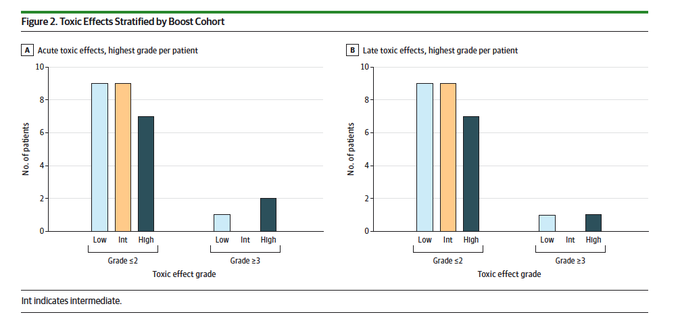
Protocol-specified MTD was not exceeded. Non-heme acute and late grade 3+ toxicities were 11% and 7%, respectively. No grade 3+ toxicities were seen in the intermediate-dose boost cohort. Two deaths in the high-dose boost cohort.
Local control was 74%, 86%, 100% in the low, intermediate, and high-dose cohort! Two-year overall survival was 30%, 76%, and 56%, respectively.
We think that this regimen holds promise as a new backbone for chemo-radiation that utilizes adaptive SABR to 70 Gy in 15 treatments with chemo as it appears to be very effective and safe.
We think HyCRT-SABR with concurrent chemo-radiotherapy and a PET-guided adaptive SABR boost technique/approach to 70 Gy in 15 treatments holds promise as an well-tolerated regimen with excellent loco-regional disease control that, if integrated with immunotherapy and/or other future novel therapeutics in locally advanced unresectable lung cancer, may achieve even better outcomes for our lcsm patients!
I would like to give a shoutout to all authors: Especially Trudy Wu, Elaine Luterstein, Patrick Kupelian, Minsong Cao, Stephen Tenn, Michael Steinberg for their support of this radio-oncology dose-escalation study for unresectable stage II-III NSCLC using a novel HyCRT-SABR approach.
Humbly, I would like to take a moment to acknowledge many of my mentors through the years (not all included): Albert Koong, Edward Kim, Annette Walker, Quynh Thu Le, Sarah Donaldson, Richard Hoppe, Patrick Kupelian and many others. You inspired me to do better for patients.
Most importantly, from the bottom of my heart, I would like to sincerely thank the patients, caregivers, family that entrusted us with their family members afflicted with lung cancer who we had the privilege to take care of and who participated in this trial.
Without you, we would not have been able to advance the science for future patients’ benefit.
Let the questions and constructive critiques begin! Open to any and all of them in order to take this approach to the next level for our patients!”
Source: Percy Lee/X
