
CARsgen Therapeutics has announced the launch of an Investigator-Initiated Trial for KJ-C2219
On Dec 31st, CARsgen Therapeutics has announced the launch of an IIT in China for KJ-C2219, an allogeneic CAR T-cell therapy targeting CD19/CD20.
This trial aims to assess the efficacy of KJ-C2219 in treating relapsed/refractory B-cell non-Hodgkin lymphoma (R/R B-NHL).
KJ-C2219 is developed using CARsgen’s THANK-u Plus platform, which is intended for treating hematologic malignancies and autoimmune disorders. This initiative reflects CARsgen’s commitment to advancing innovative CAR T-cell therapies for various cancers and diseases.
About THANK-u Plus platform
CARsgen has introduced the THANK-u Plus platform, an advanced iteration of its proprietary THANK-uCAR allogeneic CAR-T technology, aimed at addressing the influence of NKG2A expression levels on treatment effectiveness.
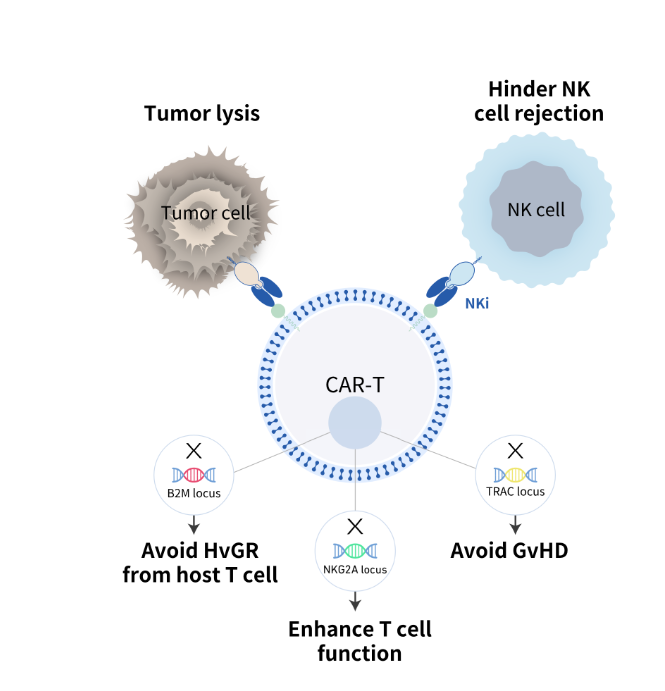
This platform maintains consistent expansion of NK cells, regardless of NKG2A expression variations, and shows significantly enhanced expansion compared to the original THANK-uCAR.
Preclinical research indicates that THANK-u Plus provides superior antitumor efficacy when NK cells are present, outperforming its predecessor. The platform has demonstrated robust antitumor activity in allogeneic BCMA or dual-targeting CD19/CD20 CAR-T cells, highlighting its potential for developing a range of allogeneic CAR-T therapies.
About CARsgen Therapeutics Holdings Limited

CARsgen is a biopharmaceutical firm operating in China and the U.S., dedicated to pioneering CAR T-cell therapies for hematologic malignancies and solid tumors. The company has built a comprehensive research and development infrastructure for CAR T-cell therapies, encompassing target discovery, innovative product development, clinical trials, and large-scale manufacturing.
CARsgen is committed to overcoming challenges faced by existing CAR T-cell treatments through novel technologies and a diverse product pipeline. Their goals include improving safety profiles, enhancing efficacy against solid tumors, and reducing treatment costs. Ultimately, CARsgen aims to establish itself as a leader in biopharmaceuticals by providing innovative and differentiated cell therapies for cancer patients worldwide.
Further Reading:
Immunotherapy for Cancer: An Overview
History of Immunotherapy: Cancer Treatment
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
