John Gordon, Vice president of
“Dual ‘ON/OFF’-switch CARs controlled by two clinically approved drugs. Longer & safer CAR T cell therapy from Melita Irving & Co. Breaking Open Access Study at Proceedings of the National Academy of Sciences.
T cells can be gene-modified with chimeric antigen receptors (CARs) to recognize and directly kill tumor cells. While CAR T cell therapy has been approved to treat some B cell malignancies, for nonhematological solid tumors toxicity and limited efficacy remain important obstacles. One approach to improve safety and T cell persistence is to incorporate an ON- or OFF-switch directly into the CAR that can be remotely regulated by administration of a small molecule.
Greta Maria Paola Giordano Attianese, Sailan Shui, Elisabetta Cribioli, Mélanie Triboulet, et al have developed an ON-CAR that can be induced by venetoclax as well as an all-in-one ON/OFF-CAR down-regulated by lenalidomide. Both of these small molecules are clinically approved and the switch components are of human origin, thus reducing the risk of immunogenicity.
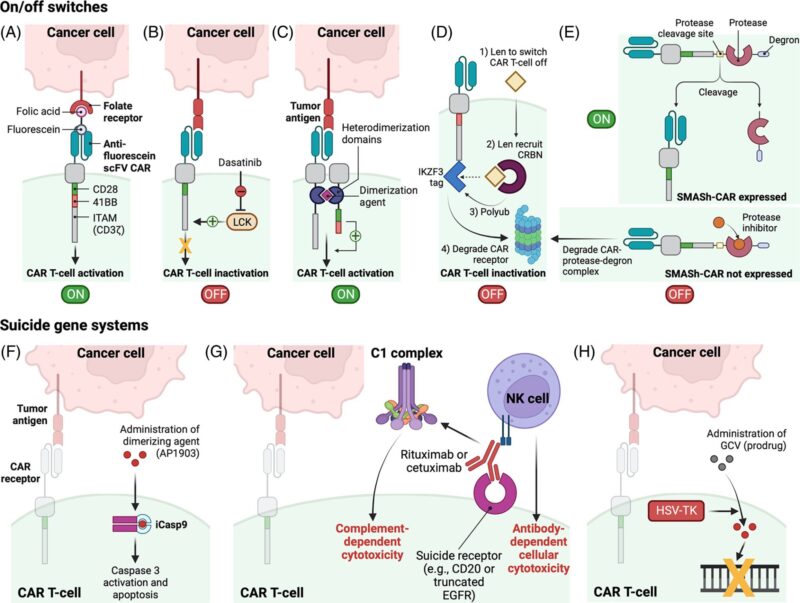
FIGURE | Upper: Other examples of on–off switchable chimeric antigen receptor T-cell (CAR-T) cells | (A) CAR-T-cells with on–off switches mediated by small-molecular adapter ligands. (B) Small molecule inhibitors such as dasatinib are able to inhibit downstream CD3ζ signaling to prevent CAR-T-cell activation. (c) CAR-T cells with on–off switches mediated by chimeric antigen receptor (CAR) subunit-dimerizing agents (e.g., lenalidomide on-switch split CAR). (D) Lenalidomide off-switch degradable CARs are degraded in the presence of lenalidomide or other thalidomide analogs. (E) Small molecule-assisted shutoff chimeric antigen receptor (SMASh-CAR) system utilizing protease inhibitors to control CAR protein expression | Lower: Suicide gene systems (an alternate strategy to overcome CART-cell toxicities) utilizing (F) inducible caspase 9 (iCasp9) to trigger apoptosis, (G) antibody-dependent cellular cytotoxicity and/or complement-dependent cytotoxicity, and (H) herpes simplex virus thymidine kinase (HSV-TK) Mut 2 gene-dependent disruption of DNA replication | Taken from Jonas Paludo & Co.
 ”
”
Authors: Matthew Ho, Saurabh Zanwar, Jonas Paludo