The CCTG NE2 STOPNET Study: Exploring the Role of Somatostatin Analogues in Neuroendocrine Tumor Treatment
The Australasian Gastro-Intestinal Cancer Trials Group is spearheading the international STOPNET trial, with the Canadian arm conducted by the CCTG. This trial aims to gather data that will inform the feasibility and design of a larger subsequent clinical trial, as well as influence public health decisions and treatment guidelines for neuroendocrine tumors (NETs).
The trial has been provided by the Canadian Neuroendocrine Tumour Society (CNETS) and the Canadian Institutes of Health Research (CIHR), with additional support from the Canadian Cancer Society, facilitating Canadian participation in this important international effort.
The CCTG NE2 trial has been awarded $260,101 in the Spring CIHR grants announcement to support Canadian participation in the STOPNET Stopnet clinical trialinternational trial, a Randomized Study of Cessation of Somatostatin Analogues after Peptide Receptor Radionuclide Therapy in Mid-Gut Neuroendocrine Tumours.
The CCTG NE2 STOPNET neuroendocrine study is focusing on a pivotal question for patients with neuroendocrine tumors (NETs): whether somatostatin analogues (SSAs) are necessary after targeted therapy.
The study aims to evaluate the effectiveness of continuing versus stopping somatostatin analogues (SSAs) during and after peptide receptor radionuclide therapy (PRRT) for patients with neuroendocrine tumors (NETs). Participants will be randomly assigned to either continue or cease SSA injections following the initiation of PRRT.
This research is significant because it explores the possibility that stopping SSA injections could control the cancer as effectively as the standard approach, while potentially reducing side effects for patients.
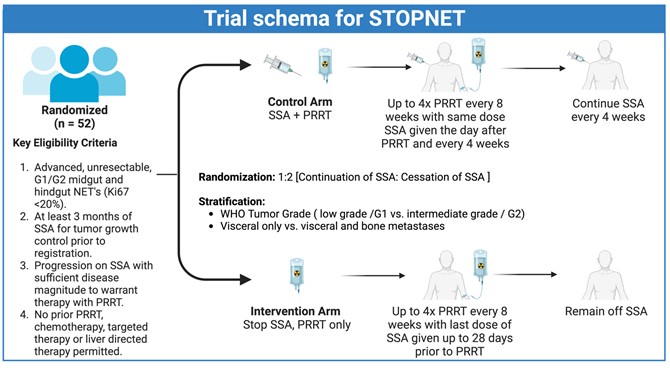
This study not only seeks to advance the understanding of treatment options for neuroendocrine tumors but also emphasizes the importance of patient-centered outcomes. By involving patients in the decision-making process and assessing factors such as quality of life and psychological impact, the CCTG NE2 STOPNET study aims to ensure that the treatment approaches developed are aligned with the needs and preferences of those affected by NETs.
Eligible participants include adults over 18 with grade 1 or 2 midgut, hindgut, or pancreatic NETs that are inoperable, have been on SSA injections for at least three months, and whose tumors have progressed requiring PRRT.
The co-chairs of the CCTG NE2 STOPNET neuroendocrine study are Dr. Jonathan Loree, an oncologist at BC Cancer, and Dr. Rachel Goodwin, an oncologist at Ottawa Hospital, who oversee the trial’s design and implementation. Chris O’Callaghan serves as a Senior Investigator, providing leadership and expertise throughout the clinical trial process. Additionally, Haydn Bechthold acts as the CCTG Patient Representative, offering valuable insights to ensure that patient experiences and outcomes are prioritized throughout the study.
Here are some insights from the trial members:
Dr Jonathan Loree, the NE2 study co-chair and BC Cancer Oncologist:
“NE2 addresses an important question for patients and the healthcare system of whether we can reduce treatment burden by stopping SSAs after PRRT has been completed.”
“The STOPNET clinical trial will help us determine whether it’s necessary to continue somatostatin analogues (SSAs) for all patients with neuroendocrine tumors (NETs) receiving peptide receptor radionuclide therapy (PRRT). If we are able to stop SSAs in patients without hormone secreting tumors, this will reduce patient treatment burden and result in considerable cost savings for the health care system.”

Dr Rachel Goodwin, the NE2 study co-chair and Ottawa Hospital Oncologist
“NE2 will also assess important patient-related outcomes such as quality of life and psychological impact of SSA cessation.”
“STOPNET is one of the first international pilot study designed to assess feasibility of SSA cessation after PRRT treatment. This phase II pilot study will allow the research community to design a successful phase III study.”

Haydn Bechthold, CCTG Patient Representative:
“NE2 represents a significant opportunity for patients with neuroendocrine tumours to potentially reduce their treatment burden and aims to help patients avoid unnecessary side effects while maintaining their quality of life. I find it very encouraging to see the study focus on outcomes that directly impact patients, including quality of life and psychological well-being.”

Chris O’Callaghan, CCTG Senior Investigator:
“It is unclear whether patients with neuroendocrine tumours requiring Peptide Receptor Radionuclide Therapy (PRRT) gain additional benefit from continued use of somatostatin analoque (SSA) drugs, or whether their side effects, expense and inconvenience can be safely avoided. The STOPNET clinical trial is a first step toward addressing what is an important question for all of patients, physicians and healthcare funders.”
“It’s baby steps, it never ends, but it can be quite rewarding if you make progress bit by bit by bit.”

Stay tuned by visiting oncodaily.com


