Rebecca Shatsky shared a post by Paolo Tarantino on X, adding:
“Love my good friend Paolo Tarantino’s explanation of Her2 Ultra Low!
A must read!”
Quoting Paolo Tarantino’s post:
“What is HER2 ultra low breast cancer, and why should you care?
A thread:
First, a little history.
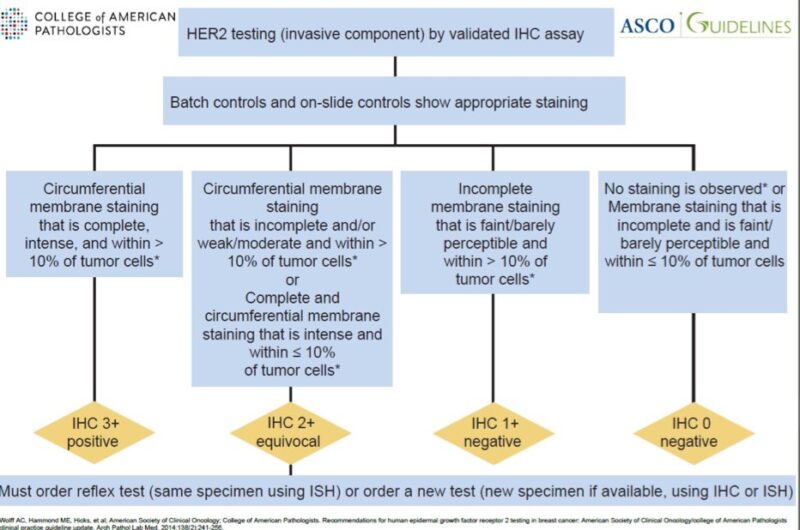
The ASCO/CAP guidelines from 2007 defined HER2 IHC 0 as ‘absence of HER2 staining’.
In truth, it did not make much difference if the tumor was 0 or 1+, since both were considered ‘negative’ for HER2 protein expression.

The 2013 update changed the definition of HER2 IHC 0 to: -no staining OR -incomplete, faint/barely perceptible staining in ≤10% of cells.
This change still had no clinical impact, since only 3+ and 2+/ampl cases were candidate for anti-HER2 therapy.
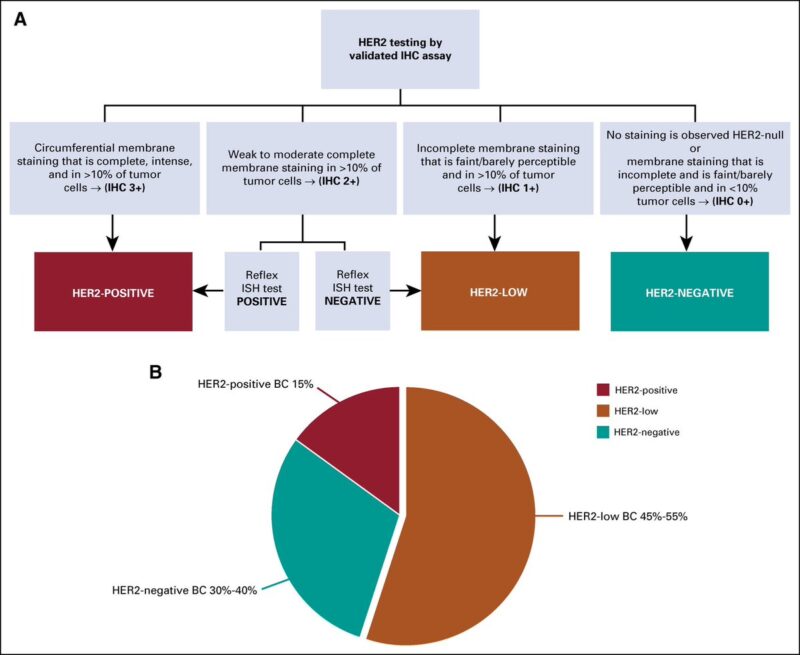
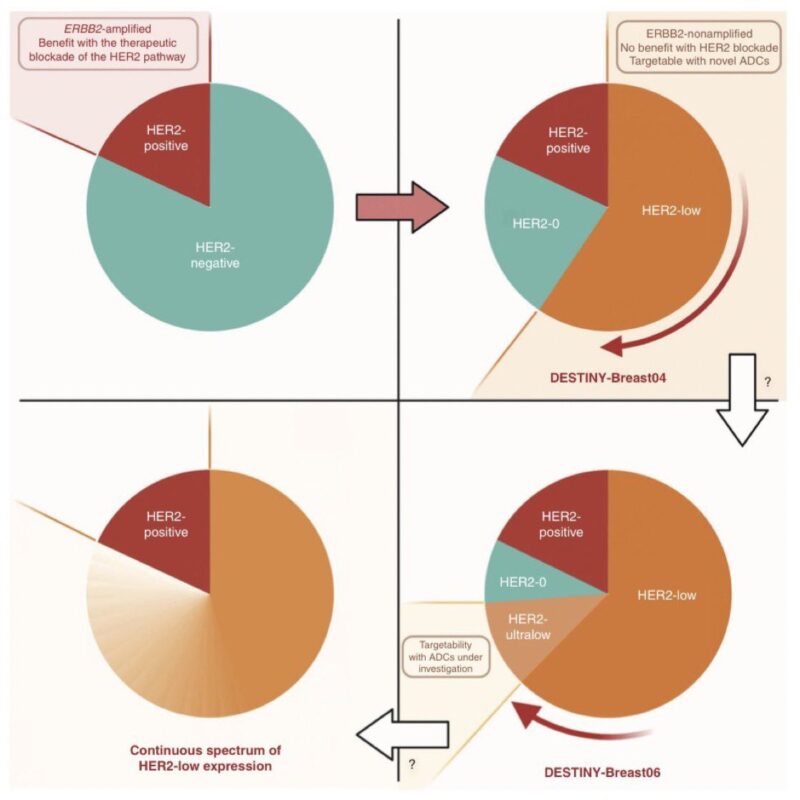
Everything changed between 2018-2022 T-DXd showed to prolong OS in HER2low metastatic breast cancer, becoming a new actionable entity What about HER2 ultralow?
Still no trace: all pts with HER2 0 mBC were excluded from DB04, irrespective of absence or weak expression.
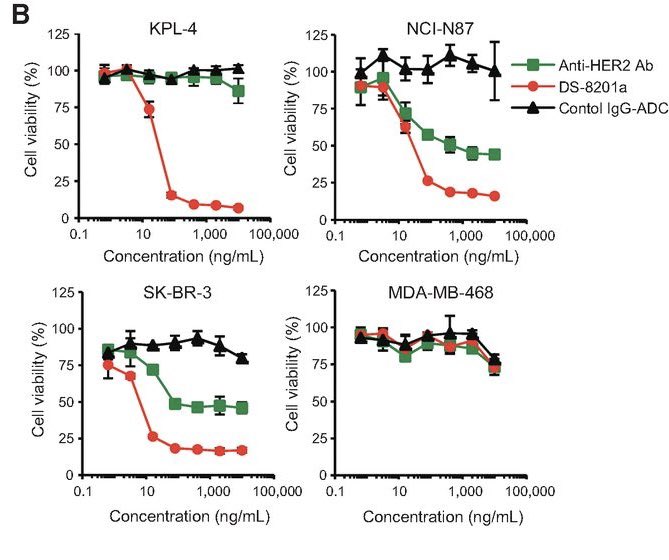
Not for long, though: a preclinical study had shown that T-DXd required only minimal HER2 expression to be active, providing the rationale to test it in some of the patients with HER2-0 disease.
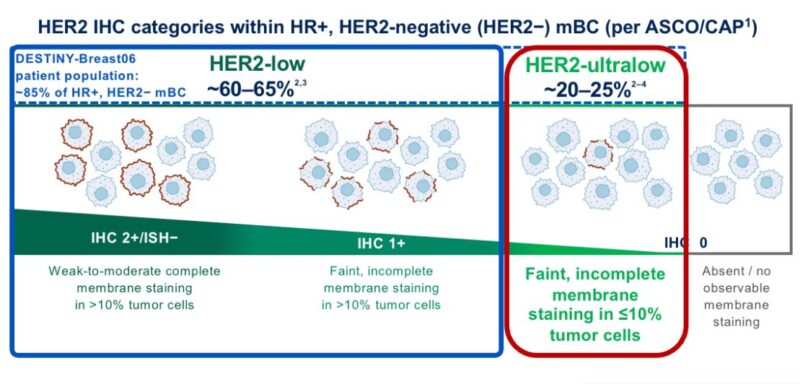
those with incomplete, faint staining in <10% of cells, aka, HER2-ultralow!
But what is the incidence of HER2-ultralow tumors?
There are only few studies answering this question.
The available data suggest that more than half of traditional HER2 IHC 0 tumors can be considered HER2-ultralow.
Thus, ≃20% of patients with mBC.
The study that tested T-DXd in patients with HER2-ultralow breast cancer is DESTINY-Breast06.
The trial included 153 patients with HR+ mBC and centrally confirmed HER2-ultralow status, randomized to T-DXd vs. chemotherapy (exploratory analysis).
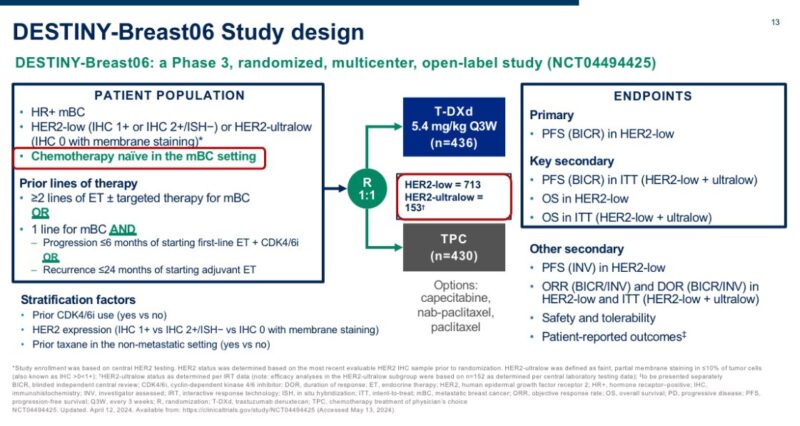
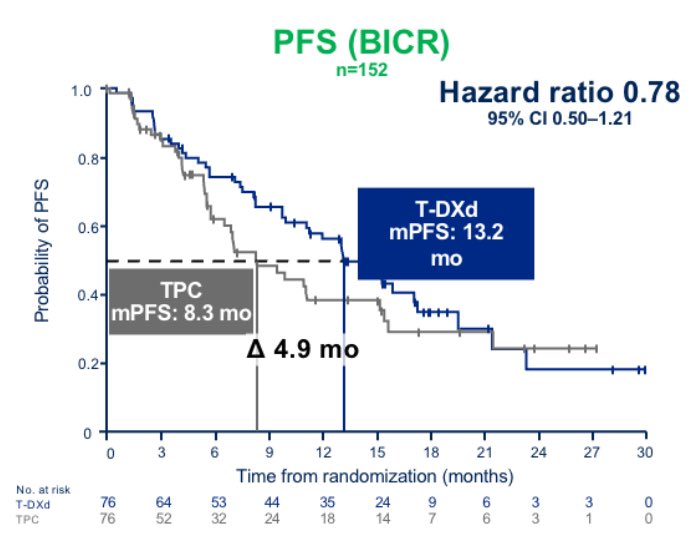
In this population, T-DXd showed a numerical improvement in PFS that appeared extremely consistent with what observed in patients with HER2-low disease >1 year PFS with T-DXd in patients traditionally considered HER2-0 was quite remarkable to see, and unexpected for many.
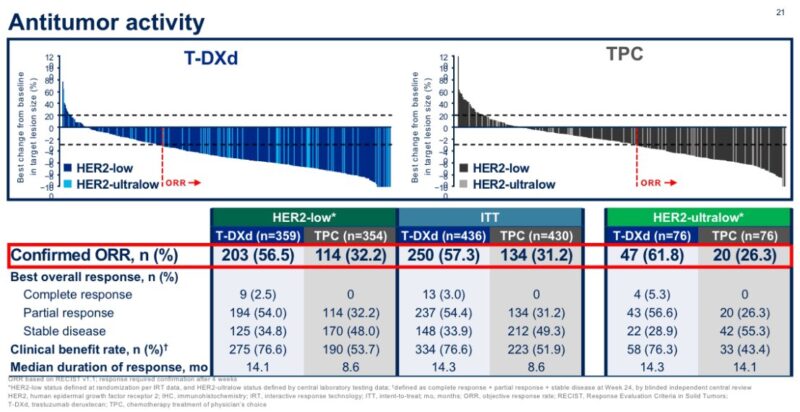
Even more strikingly, T-DXd showed higher response rate in patients with HER2-ultralow (ORR 61%) than HER2-low disease (56%) further confirming a clear benefit in this population.
Based on these data, HER2-ultralow can now also be considered actionable in breast oncology, at least for HR+ mBC And how do we now call HER2-0 tumors that are NOT ultralow?
The agreement within the first ESMO consensus was to call them ‘HER2-null’.
To produce data with T-DXd in TNBC and in the ‘HER2-null’ setting, a new trial was initiated: DESTINYBreast15, a phase 3b trial that will enroll 250 patients with HER2-0 or HER2-low mBC.
What next?
New assays!
HER2 IHC categories have never been fine tuned for HER2 ADCs. Quantitative assays in development may instead refine (and expand) our ability to select patients for ADCs We hope to show some data soon.
Stay tuned.”
Source: Rebecca Shatsky/X and Paolo Tarantino/X
Rebecca Shatsky is an Associate Professor at UC San Diego Health, Breast Medical Oncology Co-Team Leader, Scientific Director of the Inflammatory and Triple Negative Breast Cancer Program. She is a member of the NCCN guideline panel for Genetic/High-Risk Assessment of Breast, Ovarian and Pancreatic Cancer and the panel for the treatment of Cancer-Associated Pain.
Her work has been published in JAMA Oncology, Cancer Cell and Molecular Cancer Therapeutics, among others. Dr. Shatsky is a member of the American Association for Cancer Research and the American Society of Clinical Oncology.
Paolo Tarantino, MD researcher, holds positions at both the European Institute of Oncology in Milan, Italy, and the Dana-Farber Cancer Institute in Boston, MA, Specializing in breast cancer.
Currently, Dr. Tarantino is pursuing an advanced research fellowship at Dana-Farber Cancer Institute and Harvard Medical School, concurrently working towards a PhD in clinical research at the University of Milan.
His research focuses on exploring the HER2 oncoprotein, investigating the emerging HER2-low subgroup of breast tumors, and developing novel antibody-drug conjugates targeting every subtype of breast cancer.
With a robust publication record exceeding 50 papers on breast cancer, he is recognized as a leading expert in the field. Dr. Tarantino’s dedication and innovative approach contribute significantly to the advancement of breast cancer treatment and research.


