Tislelizumab (Tevimbra) is a new immunotherapy drug designed to help the body’s immune system fight cancer more effectively. It has received FDA approval for treating certain advanced cancers, including esophageal and gastric cancers. If you or a loved one has been diagnosed with one of these cancers, understanding this drug can help you make informed treatment decisions.
What Is Tislelizumab and How Does It Work?
Tislelizumab is a type of immunotherapy called a PD-1 inhibitor. Normally, cancer cells use a protein called PD-L1 to hide from the immune system. By blocking the PD-1 protein on T cells, Tislelizumab prevents cancer cells from evading immune detection, allowing the immune system to attack and destroy them. Unlike some other PD-1 inhibitors, Tislelizumab is designed to work more efficiently with fewer side effects by reducing unnecessary immune cell depletion.
What Cancers Does Tislelizumab Treat?
Tislelizumab is an immunotherapy drug that helps the immune system fight cancer. The FDA approved it on December 27, 2024, for advanced stomach and gastroesophageal cancer (HER2-negative, PD-L1 positive) with chemotherapy. It was also approved on March 4, 2025, for advanced esophageal cancer (ESCC)—first for previously treated patients and later as a first-line treatment with chemotherapy.
Beyond the U.S., China approved Tevimbra in 2022 for lung and nasopharyngeal cancer, while the European Union (EU) approved it in 2023 and 2024 for esophageal and lung cancer treatments. These approvals highlight its growing role in treating advanced cancers.

Learn How Does Immunotherapy Work on OncoDaily.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Tevimbra was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.
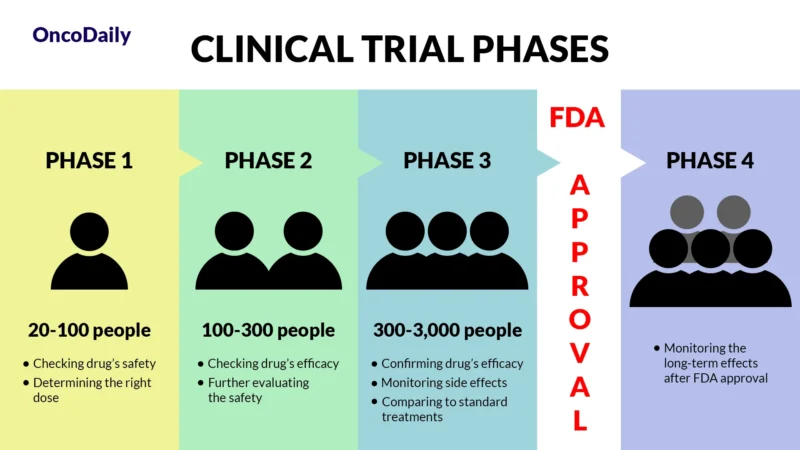
What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it is both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
Clinical trials have shown that Tislelizumab is effective in treating certain types of cancer, with encouraging results:
In the RATIONALE-302 trial for second-line treatment of esophageal squamous cell carcinoma (ESCC), patients who received Tislelizumab had a median overall survival (OS) of 8.6 months, compared to 6.3 months for those who only had chemotherapy. This treatment reduced the risk of death by 30%.
In the RATIONALE-306 trial for first-line treatment of ESCC with chemotherapy, patients treated with Tislelizumab had a median OS of 17.2 months, compared to 10.6 months for chemotherapy alone, reducing the risk of death by 34%.
In the RATIONALE-305 trial for gastric and gastroesophageal junction (GEJ) adenocarcinoma, when combined with chemotherapy, the median OS was 15.0 months, compared to 12.9 months with chemotherapy alone. Additionally, the response rate was 48% for Tislelizumab plus chemotherapy, compared to 41% with chemotherapy alone.
These results show that Tislelizumab can improve survival and response rates, making it a promising option for treating advanced cancers.
Learn more about Immunotherapy for Cancer: An Overview on OncoDaily.
What Other Trials Are Ongoing?
Tislelizumab is being studied for the treatment of more types of cancer to see how it might help patients in different situations.
For non-small cell lung cancer (NSCLC), researchers are looking at whether Tevimbra can be used as a neoadjuvant therapy—meaning it might be given before surgery to shrink tumors and improve outcomes.
In head and neck squamous cell carcinoma (HNSCC), studies are testing its use as part of first-line combination treatments to improve how well the treatment works when given alongside other therapies.
For pancreatic cancer, researchers are exploring whether Tevimbra could be helpful as a neoadjuvant therapy, similar to its use in lung cancer, to improve treatment before surgery.
In bladder cancer, Tislelizumab is being investigated for its role in perioperative treatment, which involves using the drug before or after surgery to improve survival chances.
These studies aim to expand the potential of Tevimbra, helping it reach more patients and improve outcomes across different cancers.
What Can You Expect During Treatment?
Tislelizumab is administered as an intravenous (IV) infusion every three weeks. The first infusion typically takes about 60 minutes, but if tolerated well, subsequent doses may be shortened to around 30 minutes. To reduce the risk of infusion reactions, patients may receive premedication such as antihistamines or steroids before each treatment.
During the infusion, healthcare professionals will closely monitor patients for any immediate reactions, such as fever, chills, or difficulty breathing. After the infusion, patients will remain under observation for a short period to ensure there are no delayed side effects.
Throughout the treatment, regular check-ups, blood tests, and imaging scans may be scheduled to track progress and assess how well the therapy is working. Some patients may experience side effects, such as fatigue, nausea, rash, or immune-related reactions, which should be reported to the healthcare team.
It is important to follow all medical advice, stay hydrated, and maintain open communication with the care team to manage any side effects effectively and maximize the benefits of treatment.
Side Effects and Their Management
Tevimbra is a treatment that works by helping the immune system fight cancer. While it can be very effective, it may also cause side effects, which vary from person to person.
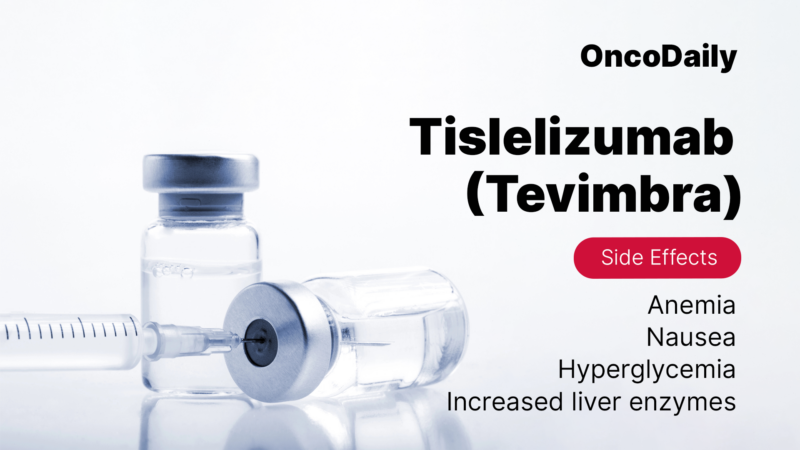
Common side effects of Tevimbra include fatigue (feeling very tired), anemia (low red blood cell count), muscle pain, cough, nausea, diarrhea, and loss of appetite. Some people may also experience changes in liver enzymes, high blood sugar, thyroid problems, and skin rashes. These side effects are usually manageable with the right care, and doctors will monitor them closely.
Less common side effects might include shortness of breath, dizziness, joint pain, and kidney problems. In some cases, the immune system might attack healthy organs, causing issues like lung problems (pneumonitis), liver inflammation (hepatitis), or intestinal issues (colitis). If this happens, it’s important to get medical attention quickly.
In rare cases, there could be more serious side effects like severe allergic reactions, heart inflammation (myocarditis), or nerve problems. These require immediate treatment.
To manage these side effects, doctors will regularly monitor patients during treatment, provide supportive care, and may adjust the treatment dose if needed. Early recognition and prompt treatment are key to preventing more serious complications and making sure the treatment works as effectively as possible. It’s important to stay in touch with your healthcare team and report any unusual symptoms right away.
What to Expect Long-Term?
Tislelizumab is not a cure but can help extend survival and improve the quality of life for many patients. Over time, some patients may develop resistance to the drug. If the cancer progresses, doctors may recommend alternative treatments or combination therapies.
What Should You Avoid During Treatment?
During treatment with Tislelizumab, patients should avoid certain things to ensure the best results and minimize risks.
Immunosuppressive medications, such as corticosteroids or other drugs that weaken the immune system, should be avoided, as they can reduce the effectiveness of the treatment.
Patients should also avoid live vaccines, including the measles, mumps, and rubella (MMR) vaccine, because they use weakened viruses that can cause infections due to the immune system being boosted by the treatment.
It is also important to limit alcohol and smoking, as both can worsen side effects and reduce the treatment’s effectiveness.
Finally, patients should always inform their doctor about any other medications or supplements they are taking, whether prescription, over-the-counter, or natural. Some of these can interact with the treatment and impact its success. By following these guidelines, patients can help ensure the treatment works as effectively as possible while reducing unwanted side effects.

You can read more about Side Effects of Cancer Immunotherapy on OncoDaily.
Real-Life Effectiveness
While clinical trials show impressive survival benefits, real-world data are still being collected. Many patients have experienced significant tumor shrinkage and prolonged survival. Ongoing studies continue to refine its role in cancer treatment.
Looking Ahead – The Future of Tislelizumab
The future of Tevimbra is very promising, with ongoing research exploring new ways it can help in cancer treatment.
Researchers are studying how Tevimbra might be effective for other types of cancer, such as lung, bladder, and pancreatic cancers. They’re also testing combination therapies, where Tevimbra is used alongside other immunotherapies, targeted therapies, or chemotherapy to improve results. There is also a focus on personalized medicine, which involves identifying specific markers in patients to predict who will benefit most from Tislelizumab treatment.
Tislelizumab represents a significant step forward in treating certain advanced cancers. Staying informed and involved in the treatment process can help make the best decisions for health and well-being.
If you’re a healthcare provider, access the professional version here.
Written by Mariam Khachatryan, MD