Linvoseltamab is a recently approved cancer treatment that represents a new wave of innovation in targeted immunotherapy. As a bispecific antibody, it is designed to engage the immune system in a highly specific way, helping the body fight cancer more effectively. Its approval marks an important step forward in the treatment of certain difficult-to-treat malignancies. This article will explore Linvoseltamab-gcpt’s uses in cancer, possible side effects, recommended dosage, treatment expectations, and more.
Which company produced Linvoseltamab?
Linvoseltamab-gcpt, marketed under the brand name Lynozyfic, was developed by Regeneron Pharmaceuticals, Inc., a U.S.-based biotechnology company known for its cutting-edge work in immunotherapy and antibody development. Founded in 1988 and headquartered in Tarrytown, New York, Regeneron has grown into one of the world’s leading biopharmaceutical companies, responsible for therapies across a broad spectrum of diseases including cancer, inflammatory conditions, cardiovascular disorders, and rare genetic diseases. The company is particularly recognized for its proprietary VelocImmune® technology, which has powered the discovery of numerous fully human monoclonal antibodies, including linvoseltamab.
How does Linvoseltamab work?
Linvoseltamab-gcpt is a bispecific antibody that works by directing the body’s immune system to attack cancer cells—specifically multiple myeloma cells that express a protein called BCMA (B-cell maturation antigen). Under normal conditions, BCMA is found on the surface of healthy plasma cells (a type of white blood cell), but it is overexpressed in malignant plasma cells in people with multiple myeloma. These cancerous cells often escape immune detection, allowing the disease to progress.
Linvoseltamab is designed to bind simultaneously to BCMA on myeloma cells and CD3 on T cells, a key type of immune cell. By bridging these two cells, linvoseltamab brings T cells into direct contact with the cancer cells, triggering the T cells to release cytotoxic substances that kill the tumor cells. This immune redirection mechanism helps re-activate the immune system against multiple myeloma, even in heavily pretreated or refractory cases. This targeted dual-binding approach not only enhances the precision of the immune attack but also minimizes damage to other healthy tissues, making linvoseltamab a promising therapeutic option in relapsed or treatment-resistant disease.
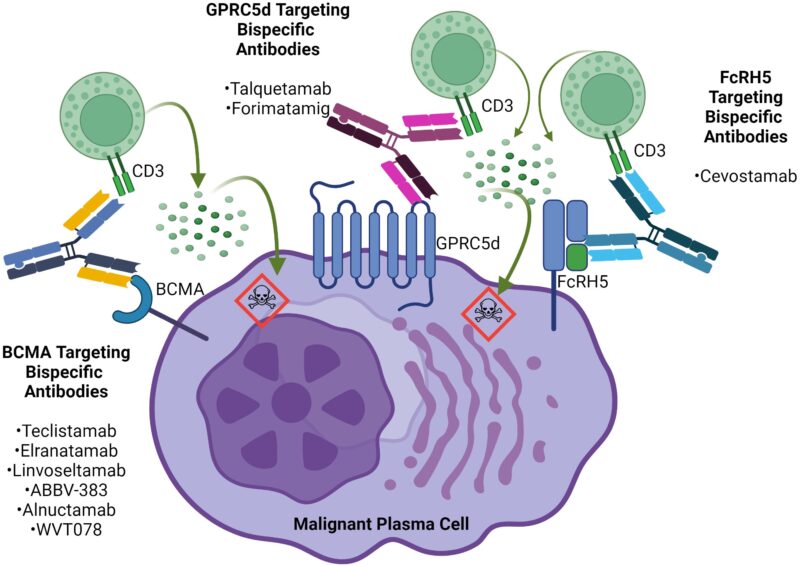
Source: Frontiers
What Cancers are treated with Linvoseltamab?
On July 2, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Lynozyfic for the treatment of relapsed or refractory multiple myeloma in adults who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
This milestone marks the approval of the first BCMAxCD3 bispecific antibody in its class, offering a new immunotherapy option for patients with limited remaining treatment choices. The FDA’s decision was based on encouraging evidence of benefit, and continued approval may depend on further confirmation in ongoing studies. Lynozyfic expands the landscape of targeted therapies in multiple myeloma and reflects ongoing innovation in immune-based cancer treatment.

Learn more about Multiple Myeloma: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
What research is behind the approval?
The approval of Linvoseltamab-gcpt (Lynozyfic) for relapsed or refractory multiple myeloma was based on results from the phase 1/2 LINKER-MM1 trial, which showed an overall response rate of 70% and a promising duration of response, with 72% of patients maintaining their response at 12 months. The study included patients heavily pretreated but with no prior BCMA-targeted therapies.
Supporting evidence was also published in the Journal of Clinical Oncology in June 2024, detailing results from a phase I/II trial that included 117 patients treated with the 200 mg dose. The ORR in this group was 71%, and 50% achieved a complete response or better. The median duration of response was 29.4 months. Among 104 patients treated with 50 mg, the ORR was 48%, with 21% reaching a complete response or better.
In terms of safety, cytokine release syndrome (CRS) was reported in 46.2% of patients, with Grade 3 CRS in 0.9%. Neurologic toxicity, including ICANS, occurred in 7.7% of patients (2.6% each for Grades 1, 2, and 3). Neutropenia was seen in 42.8% (including 23.1% Grade 4), and infections were reported in 74.4% of patients, though infection rates declined over time. Due to these risks, the drug carries a boxed warning and is only available through the Lynozyfic REMS program.
Treatment begins with step-up doses of 5 mg (Day 1), 25 mg (Day 8), and 200 mg (Day 15), followed by weekly 200 mg doses for 10 weeks. If tolerated and a very good partial response (VGPR) or better is achieved and sustained after Week 24, dosing may be reduced to every four weeks.

Linvoseltamab Side Effects and Its Management
Linvoseltamab-gcpt (Lynozyfic) is a bispecific antibody therapy that offers a new approach for treating relapsed or refractory multiple myeloma. Like many powerful immunotherapies, it can lead to side effects that vary in severity and frequency. Understanding these effects and how to manage them is key to ensuring patient safety and treatment success.
Common Side Effects
The most frequently reported side effects with Linvoseltamab-gcpt are mild to moderate in severity and often manageable with supportive care. One of the most common immune-related effects is cytokine release syndrome (CRS), which may present with fever, fatigue, chills, shortness of breath, or low blood pressure, typically occurring shortly after starting treatment. Infusion-related reactions (IRRs) are also commonly observed and may include fever, rash, nausea, or dizziness during or after the infusion.
In addition to immune-mediated symptoms, patients may develop blood-related side effects such as low levels of neutrophils (neutropenia), red blood cells (anemia), or platelets (thrombocytopenia). These changes can increase the risk of infections, fatigue, or bleeding. Other frequently observed symptoms include musculoskeletal pain, headache, cough, diarrhea, and respiratory infections like pneumonia or upper respiratory tract infections.
Less Common but Serious Side Effects
Although less frequent, some side effects require urgent medical attention. One such event is immune effector cell-associated neurotoxicity syndrome (ICANS), which can cause confusion, difficulty speaking, seizures, or more severe neurological symptoms. These events typically occur in the early phase of treatment and require close observation.
Serious infections may also occur, especially in patients with prolonged neutropenia. These can include bacterial, viral, or fungal infections that may become life-threatening if not addressed promptly. Liver-related toxicity, marked by elevated liver enzymes, has been observed in some patients, particularly in the context of CRS. Rare but potentially fatal immune complications, such as hemophagocytic lymphohistiocytosis (HLH)-like syndromes, have also been reported in the broader class of T-cell engaging therapies.
Managing Side Effects
To reduce the risk of early immune reactions like CRS and IRRs, premedication is commonly used. This includes corticosteroids, antihistamines, and antipyretics before each dose during the initial treatment phase. If CRS or an infusion reaction does occur, the infusion may be paused and resumed at a slower rate once symptoms resolve. Supportive measures like intravenous fluids, oxygen, or medications such as tocilizumab (an IL-6 receptor blocker) may be used in more severe cases.
Neurotoxicity such as ICANS requires immediate interruption of therapy. If symptoms progress, high-dose corticosteroids and consultation with neurology are typically recommended. Treatment is resumed only after symptoms resolve, and in severe cases, the drug may be permanently discontinued.
Blood count abnormalities are managed by regular monitoring and temporary treatment interruption if levels fall below certain thresholds. In some cases, patients may receive growth factors to stimulate blood cell production. For infections, clinicians may prescribe preventive antibiotics, antifungals, or antivirals, and treat any active infections aggressively. Liver enzyme elevations generally resolve with temporary drug interruption and close monitoring. If liver function improves, treatment may be resumed, potentially at a reduced dose.
What is the Recommended Dosage of Linvoseltamab?
Linvoseltamab-gcpt is available in two strengths: 5 mg in a 2.5 mL vial and 200 mg in a 10 mL vial. It is used to treat adults with relapsed or refractory multiple myeloma who have previously undergone at least four different treatment regimens, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.
Treatment begins with a step-up dosing schedule to help reduce side effects. The first dose on day 1 is 5 mg intravenously, followed by 25 mg on day 8. The full treatment dose of 200 mg is given on day 15. Starting one week after the full dose (week 4), patients receive 200 mg once weekly for a total of 10 weekly doses. After this phase, dosing shifts to every two weeks from weeks 14 to 23.
If a patient achieves and maintains a very good partial response (VGPR) or better at or after week 24, the dosing interval may be extended to every four weeks. Treatment continues on this schedule until disease progression or unacceptable toxicity occurs. The timing between doses is carefully spaced: weekly doses are at least 5 days apart, biweekly doses at least 10 days apart, and every-four-week doses at least 24 days apart to optimize safety and effectiveness.
How is Linvoseltamab administered?
Linvoseltamab-gcpt is prepared by diluting the appropriate dose in a saline (0.9% NaCl) infusion bag. The drug comes in clear to slightly yellow solutions, which should be inspected before use to ensure no cloudiness or particles are present. Any vial showing discoloration or particulate matter must be discarded. The infusion uses compatible bags made of PVC or polyolefin and tubing lined with PVC, polyethylene, or polyurethane, with a polyethersulfone filter to ensure safety. It is important not to mix Linvoseltamab-gcpt with other drugs or administer other medications through the same IV line.
After dilution, the solution should be gently mixed without shaking and used promptly. The infusion line is primed with the prepared solution and administered through a fine filter. Infusion duration varies by dose: the initial step-up doses (5 mg, 25 mg, and the first 200 mg) are infused over about four hours. The second 200 mg dose takes about one hour, and subsequent doses are infused over 30 minutes, unless side effects like cytokine release syndrome (CRS) require maintaining longer infusion times. For maintenance dosing every two or four weeks, the infusion is given over 30 minutes.
Following infusion, the line should be flushed with saline to ensure the full dose is delivered. Storage guidelines recommend keeping unopened vials refrigerated between 2–8ºC and protected from light, without freezing or shaking. Diluted solutions can be stored at room temperature for up to eight hours or refrigerated for up to 48 hours before use.
How Is Linvoseltamab-gcpt Metabolized and Eliminated?
Linvoseltamab-gcpt is metabolized through natural catabolic pathways into small peptides. It is eliminated slowly from the body, with a steady-state clearance of about 0.43 liters per day. After the last dose, the drug’s maximum concentration in the blood decreases by 97% over a median period of 77 days, reflecting its prolonged presence.
What to Avoid During Linvoseltamab Treatment?
During Linvoseltamab treatment, patients should avoid anything that might increase the risk of side effects or interfere with therapy. It’s important to avoid taking other medications or infusions through the same IV line to prevent drug interactions. Patients should also steer clear of activities or exposures that increase infection risk, as Linvoseltamab can lower blood cell counts and weaken the immune system. Avoid missing scheduled doses or delays without consulting your healthcare provider, as consistent treatment is important for effectiveness. Lastly, do not stop or change the dose of Linvoseltamab without medical advice, and avoid any over-the-counter supplements or herbal remedies unless approved by your doctor.
Linvoseltamab effectiveness over time
New data from the phase 1b LINKER-MM2 trial suggest that the combination of Linvoseltamab and bortezomib may be highly effective for patients with relapsed or refractory multiple myeloma, including those resistant to proteasome inhibitors. In this study, patients began treatment with Linvoseltamab before adding bortezomib. Among those evaluated, the overall response rate reached 79%, with 90% of responders maintaining their response for at least six months. The 6-month progression-free survival rate was also 79%.
The combination showed a manageable safety profile. The most frequently observed side effects included low blood counts and cytokine release syndrome, while neurological toxicity (ICANS) was infrequent and limited to early treatment cycles. These findings, presented at the 2025 ASCO Annual Meeting and published in the Journal of Clinical Oncology in May 2025, support further investigation of Linvoseltamab in combination regimens for earlier use in treatment.
Ongoing trials with Linvoseltamab
Regeneron Pharmaceuticals is conducting a phase 1/2 clinical trial (NCT06292780) to evaluate linvoseltamab in adults with relapsed or refractory systemic light chain (AL) amyloidosis. This study is designed to assess the safety, dosing, and preliminary effectiveness of the drug in patients whose disease has returned or no longer responds to prior treatments.
In Phase 1, a small group of participants will receive linvoseltamab to determine its safety profile and the appropriate dose. Phase 2 will expand enrollment to further evaluate how well the treatment reduces the abnormal proteins that damage organs, particularly the heart and kidneys, and to measure how long these improvements last.
Written by Mariam Khachatryan, MD
FAQ
Is Linvoseltamab chemotherapy or immunotherapy?
Linvoseltamab is a bispecific immunotherapy, not chemotherapy. It engages the immune system by connecting T cells with cancer cells that express BCMA, helping the body target and kill multiple myeloma cells more precisely.
Can Linvoseltamab be used as a first-line treatment for multiple myeloma?
Currently, Linvoseltamab is only approved for use in adults with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy.
What is the recommended dosage of Linvoseltamab?
The full treatment dose of Linvoseltamab is 200 mg subcutaneously every two weeks after initial step-up dosing. Dose modifications may be required based on tolerability or adverse events.
What is cytokine release syndrome (CRS) and how is it managed?
CRS is a common side effect of T-cell engaging therapies like Linvoseltamab, presenting with fever, chills, and low blood pressure
What is the half-life of Linvoseltamab?
Linvoseltamab has a prolonged half-life, with its concentration in the blood decreasing by 97% over approximately 77 days after the final dose.
What is ICANS and should patients be worried?
ICANS stands for immune effector cell–associated neurotoxicity syndrome, a rare but serious side effect. Symptoms may include confusion or difficulty speaking, and treatment is paused until symptoms resolve, often with corticost
What is BCMA and why is it important in multiple myeloma?
BCMA (B-cell maturation antigen) is a protein highly expressed on malignant plasma cells in multiple myeloma. Linvoseltamab targets BCMA to guide immune cells to the tumor, making it a critical therapeutic target in this cancer.


