Tebentafusp (brand name KIMMTRAK) is a first-in-class bispecific T-cell receptor (TCR) fusion protein designed to treat metastatic uveal melanoma (mUM), a rare and aggressive form of eye cancer. Unlike immune checkpoint inhibitors (such as PD-1 or CTLA-4 inhibitors), which help restore an immune response, Tebentafusp actively redirects T-cells toward the tumor.
Which company produced KIMMTRAK?
Immunocore Holdings PLC is a biotechnology company specializing in T-cell receptor (TCR) therapies for cancer, infectious diseases, and autoimmune conditions. Founded in 2008 and headquartered in Oxford, United Kingdom, Immunocore is a leader in TCR-based immunotherapy and has pioneered its proprietary ImmTAC® (Immune Mobilizing Monoclonal TCRs Against Cancer) platform. Tebentafusp (KIMMTRAK) was developed by Immunocore Holdings PLC and gained FDA approval on January 25, 2022, for the treatment of HLA-A*02:01-positive metastatic or unresectable uveal melanoma. It was the first T-cell receptor (TCR) therapy approved for a solid tumor.
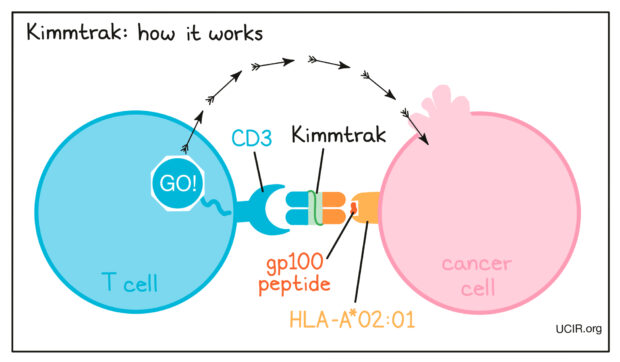
How does Tebentafusp Work?
Tebentafusp is a bi-specific protein, which means that it is double-ended and each of its ends attaches to something different. One of its ends attaches to a tiny little piece of protein called gp100, a peptide presented by HLA-A*02:01 on melanoma cells. The other end of Tebentafusp is designed to attach to CD3, which is always found on a T-cell surface. This brings a physical bridge connecting a T-cell to a melanoma cell. Cytotoxic T-cells release perforins and granzymes, leading to apoptosis (cell death) of the melanoma cells.
What Cancers are treated with Tebentafusp?
Tebentafusp/Kimmtrak is approved for the treatment of HLA-A*02:01-positive adults with unresectable or metastatic uveal melanoma. The IMCgp100-202 trial marked a significant breakthrough in the treatment of metastatic uveal melanoma (mUM), a rare and aggressive cancer with historically poor outcomes. The study compared Tebentafusp (KIMMTRAK) to standard therapies, including pembrolizumab, nivolumab, ipilimumab, and dacarbazine, and found a clear survival advantage for Tebentafusp.
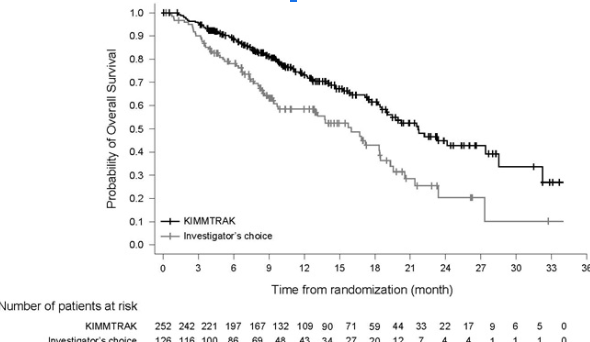
One of the most striking findings was overall survival (OS). Patients receiving Tebentafusp lived a median of 21.7 months, compared to 16 months in the control group. This translated to a 49% reduction in the risk of death, making it the first therapy to show a meaningful survival benefit in mUM. While Tebentafusp did not dramatically halt tumor progression—progression-free survival (PFS) was only slightly longer than the control group (3.3 months vs. 2.9 months)—its ability to extend life was undeniable. The objective response rate (ORR) was modest, with 9.1% of patients experiencing tumor shrinkage, and only one patient achieving a complete response. This suggests that while Tebentafusp does not always reduce tumor size significantly, it still enhances survival, likely by altering the immune response against cancer.
Despite its limitations in controlling disease progression, Tebentafusp represents a major advancement in the treatment of metastatic uveal melanoma, a disease that previously lacked effective systemic options. The findings from this trial establish Tebentafusp as the new standard of care for HLA-A*02:01-positive patients, and future research may explore combination strategies to improve tumor response while maintaining its survival benefit.
Side Effects and Management
Common side effects of Tebentafusp include cytokine release syndrome (CRS), rash, pyrexia (fever), and hypotension. CRS is a serious condition that may manifest as fever, chills, trouble breathing, confusion, severe vomiting or diarrhea, fast or irregular heartbeats, and feeling light-headed or very tired. Management of CRS involves close monitoring and may require interventions such as corticosteroids and supportive care. Other side effects include increased liver enzymes (AST/ALT), fatigue, nausea, and pruritus (itching). Monitoring liver function tests and providing symptomatic treatment are essential for managing these adverse effects.
What is the Recommended Dosage of Tebentafusp?
The recommended dosing regimen for Tebentafusp/Kimmtrak is as follows:
- Day 1: 20 mcg intravenously
- Day 8: 30 mcg intravenously
- Day 15 and weekly thereafter: 68 mcg intravenously
Treatment should continue until disease progression or unacceptable toxicity occurs. Tebentafusp dosing may need adjustments based on side effects. Moderate cytokine release syndrome (CRS) can be managed with fluids and oxygen, but persistent cases may require corticosteroid premedication. Severe CRS, causing hemodynamic instability or respiratory distress, requires withholding treatment until resolved, along with IV corticosteroids. Life-threatening CRS necessitates permanent discontinuation. Skin reactions (Grade 2-3) require withholding treatment until symptoms improve, while Grade 4 reactions lead to discontinuation. Elevated liver enzymes (Grade 3-4) warrant a temporary hold, with resumption based on CRS severity. Other Grade 3 adverse reactions require withholding treatment until resolved, while Grade 4 reactions lead to discontinuation. No dosage adjustments are needed for mild to moderate renal or hepatic impairment, but severe cases have not been studied.
Precautions During Treatment
Patients receiving Tebentafusp should be monitored for signs of CRS, especially during the initial infusions. It is advisable to avoid live vaccines during treatment and to inform healthcare providers of all medications and supplements being taken to assess potential interactions. Pregnant women or those planning to become pregnant should discuss potential risks with their healthcare provider, as Tebentafusp may cause fetal harm. Pregnancy and Tebentafusb: Tebentafusp may harm a fetus, so pregnancy status should be confirmed before starting treatment. Women should use contraception during therapy and for one week after the last dose. Breastfeeding is not recommended during treatment or for a week afterward due to unknown risks.
Ongoing trials with Tebentafusp/Kimmtrak
Several ongoing clinical trials are exploring tebentafusp’s potential in treating melanoma beyond its current indications. TEBE-AM Phase 2/3 study is evaluating Tebentafusp alone and in combination with pembrolizumab for advanced non-ocular melanoma, comparing it to standard treatments. Another major trial, the ATOM study, is assessing whether Tebentafusp can reduce relapse risk in high-risk uveal melanoma when used as adjuvant therapy. Researchers are also investigating its combination with Yttrium-90 radioembolization for patients with liver metastases. Additionally, the TebeMRD study is examining its role in minimal residual disease following initial melanoma treatment. These trials aim to expand tebentafusp’s therapeutic applications and improve patient outcomes.
What is Uveal Melanoma: What do you have to know?
Uveal melanoma is the most common primary intraocular malignancy in adults, arising from melanocytes within the uveal tract, which includes the iris, ciliary body, and choroid. Though rare, it is an aggressive cancer with a high risk of metastasis, primarily to the liver. The exact cause of uveal melanoma isn’t fully understood, but it is believed to stem from genetic mutations that lead to uncontrolled cell growth. Unlike skin melanoma, which is strongly linked to sun exposure, the role of UV light in uveal melanoma is still debated. However, people with fair skin and light-colored eyes, particularly blue or green, seem to be at higher risk. Age also plays a role, as most cases are diagnosed in people over 50, with men slightly more affected than women. Certain genetic mutations, such as those in the GNAQ, GNA11, BAP1, and SF3B1 genes, are commonly associated with the disease. Additionally, individuals with ocular nevi (benign pigmented spots in the eye) or a rare condition called oculodermal melanocytosis (Nevus of Ota) have an increased risk of developing uveal melanoma.
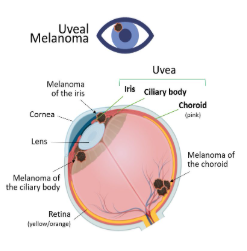
Many patients don’t experience symptoms in the early stages, making regular eye exams crucial for early detection. When symptoms do appear, they often include blurred vision, flashes of light, floating dark spots, or a visible pigmented lesion on the iris. Some may experience eye pain or increased pressure if the tumor affects intraocular structures. Uveal melanoma is often detected during routine eye exams, as it may not cause symptoms early on. Diagnosis relies on imaging techniques like ophthalmoscopy, ultrasound, and optical coherence tomography, with biopsies and genetic testing used in select cases to assess risk. Treatment depends on tumor size and spread. Small to medium tumors are often treated with radiation, such as plaque brachytherapy or proton beam therapy, to preserve vision. Larger or aggressive tumors may require surgery, including enucleation (eye removal). For metastatic cases, Tebentafusp (KIMMTRAK) has shown promise in improving survival. Since uveal melanoma commonly spreads to the liver, liver-directed therapies and clinical trials are ongoing. Regular follow-ups are crucial to monitor for recurrence or metastasis.
What Are Bispecific T-Cell Receptor (TCR) Therapies, and How Do They Differ from Traditional Immunotherapy?
Bispecific T-cell receptor (TCR) therapies, like Tebentafusp, are engineered proteins designed to simultaneously bind tumor-specific antigens and activate T-cells for a targeted immune response. Unlike traditional checkpoint inhibitors (e.g., PD-1 or CTLA-4 inhibitors), which enhance the immune system’s general ability to detect cancer, bispecific TCRs actively redirect T-cells toward tumor cells, even if the immune system has not recognized them. This dual-targeting mechanism enhances precision and efficacy, making bispecific TCRs a promising approach for cancers with limited treatment options, such as metastatic uveal melanoma.
Written by Mariam Khachatryan, MD
FAQ
How Is Tebentafusp Administered?
Tebentafusp is given as an intravenous (IV) infusion, typically once a week. The initial doses are lower (20 mcg on Day 1, 30 mcg on Day 8) to help the body acclimate and reduce severe side effects, after which the standard dose of 68 mcg is administered weekly.
Is Tebentafusp an immunotherapy drug?
Yes, it is a bispecific T-cell receptor (TCR) immunotherapy.
What Is HLA-A*02:01, and Why Does It Matter for Tebentafusp Treatment?
Tebentafusp is specifically approved for patients who test positive for the HLA-A02:01 allele. This allele allows Tebentafusp to bind to the gp100 peptide on tumor cells and redirect T-cells to attack them. Before starting treatment, your healthcare provider will typically perform a blood test to confirm you have the HLA-A02:01 variant.
Can Tebentafusp be used for cancers?
Yes, it is approved for metastatic uveal melanoma and is being studied for other cancers.
How Long Does Treatment with Tebentafusp Typically Last?
Treatment continues weekly until there is evidence of disease progression or intolerable toxicity. Some patients may remain on Tebentafusp for many months if they tolerate the infusions well and show clinical benefit, even if imaging does not indicate a major reduction in tumor size.
Are There Other Treatment Options for Metastatic Uveal Melanoma?
Yes. Historically, patients with metastatic uveal melanoma have been treated with immunotherapies like pembrolizumab or nivolumab, or targeted therapies if they have specific mutations. However, Tebentafusp is the first therapy to demonstrate a clear survival benefit in metastatic uveal melanoma, making it a new standard of care for HLA-A*02:01-positive patients.
Are Kimmtrak and Tebentafusp the same?
Yes, Kimmtrak is the brand name, while tebentafusp is the generic name of the same drug. The difference lies in the terminology: Kimmtrak refers to the marketed product, and tebentafusp is the active drug itself.
What Should I Expect During the First Few Infusions of Tebentafusp?
The first few infusions often carry the highest risk of cytokine release syndrome (CRS), which can present as fever, chills, nausea, or low blood pressure. Most patients are closely monitored in a hospital setting for at least several hours after each infusion. If CRS occurs, it may be managed with IV fluids, supplemental oxygen, or corticosteroids.
Can Tebentafusp Be Used in Combination with Other Therapies?
Research is ongoing to evaluate Tebentafusp in combination with other immunotherapies, such as checkpoint inhibitors, and with local liver-directed treatments for metastases. Clinical trials like TEBE-AM Phase 2/3 are testing these combinations, aiming to improve both survival and tumor response rates.
What Are the Most Common Side Effects Besides Cytokine Release Syndrome?
Patients frequently report rashes, itching (pruritus), elevated liver enzymes, fatigue, and nausea. Rash management typically involves topical steroids and antihistamines. Regular blood tests help monitor liver function, ensuring that any elevations in AST/ALT are addressed promptly.
How Does Tebentafusp Differ from Traditional Immune Checkpoint Inhibitors?
Traditional immune checkpoint inhibitors (like PD-1 or CTLA-4 inhibitors) work by removing the “brakes” on the immune system, allowing T-cells to become more active. Tebentafusp, on the other hand, actively redirects T-cells to the tumor by bridging gp100 on cancer cells to CD3 on T-cells, leading to a more targeted immune attack.


