Al-Ola A Abdallah, Associate Professor and Plasma Cell disorder program Director at University of Kansas Medical Center, shared a paper Dan T. Vogl and colleagues authored on X:
“Targeted interferon therapy with modakafusp alfa for relapsed or refractory multiple myeloma
Phase 1/2 clinical trial investigates the safety and efficacy of modakafusp alfa. Dose-escalation and dose-expansion phases. Modakafusp alfa delivers interferon alfa signaling to CD38+ myeloma and immune cells, leading to myeloma cell death and immune activation.
Interferon alfa has anti-myeloma activity but is limited by toxicity.
Modakafusp alfa is a fusion protein consisting of two attenuated interferon alfa-2b molecules and an anti-CD38 antibody (IgG4). This design delivers interferon alfa specifically to CD38-expressing myeloma and immune cells.
The anti-CD38 component binds to a unique epitope and does not compete with existing anti-CD38 antibodies like daratumumab or isatuximab, which stimulates type 1 interferon signaling, leading to immune cell activation and direct anti-myeloma cytotoxicity.
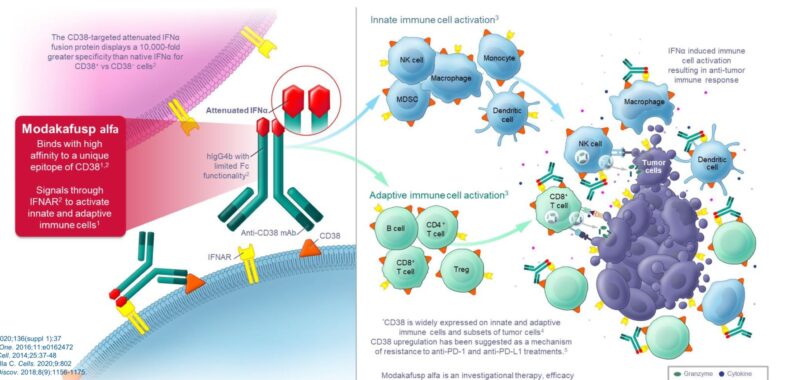
Study Design:
– Patients: R/R MM, ≥3 prior lines of therapy, refractory or intolerant to ≥1 proteasome inhibitor and ≥1 immunomodulatory drug
– Dose escalation: Modakafusp alfa was administered IV across various doses (0.001 to 6 mg/kg) and schedules (weekly, every 2 weeks, every 3 weeks, every 4 weeks).
– Dose expansion: Included cohorts with modakafusp alfa at 0.4 mg/kg Q3W, 1.5 mg/kg Q4W, with or without dexamethasone.
Primary endpoints: Safety (dose escalation) and ORR, dose expansion.
– Secondary endpoints: Pharmacokinetics, DOR, time to response, and pharmacodynamics.
– Responses were evaluated using International Myeloma Working Group criteria.
– 106 patients treated (56 in dose escalation, 50 in dose expansion). Median of 6.5 prior lines of therapy. 84% refractory to anti-CD38 antibody, and 83% triple-class refractory.
– Dosing Schedule: The Q4W schedule was found to be the most feasible. The maximum tolerated dose (MTD) was 3 mg/kg. Recommended phase 2 doses of 1.5 mg/kg Q4W and 3 mg/kg Q4W identified.
– Efficacy: At 1.5 mg/kg Q4W (n=30), the ORR was 43.3% (95% CI, 25.5-62.6). This included 1 stringent CR, 2 CRs, and 6 VGPRs. The median DOR was 15.1 months (95% CI, 7.1-26.1), and the median progression-free survival was 5.7 months (95% CI, 1.2-14).
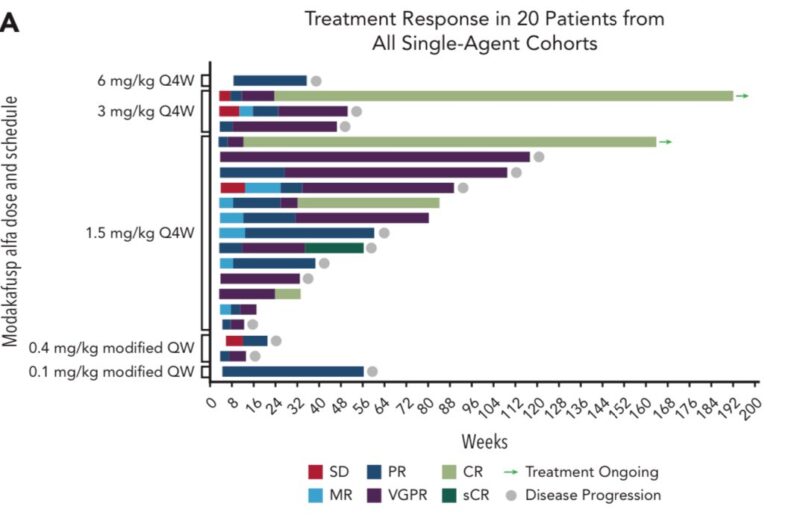
At 3 mg/kg Q4W (n=7), the ORR was 42.9%, with 1 CR and 2 VGPRs. Median DOR was 10.2 months (95% CI, 7.9 to not reached).
– Among 30 patients treated at 1.5 mg/kg Q4W, the ORR was 43.3%, with a median duration of response of 15.1 months (95% confidence interval [CI], 7.1-26.1); median progression-free survival was 5.7 months (95% CI, 1.2-14)
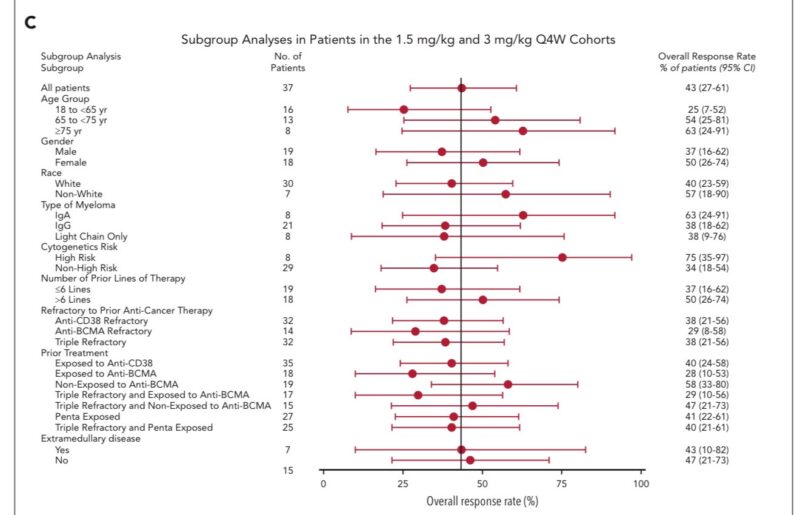
– Refractory to anti-CD38 antibodies (38% ORR), triple-class refractory disease (38% ORR), penta-exposed patients (41% ORR), and those with EMD (43% ORR).
Safety: The most common adverse events were hematologic: thrombocytopenia, neutropenia, leukopenia, and anemia.
– Grade ≥3 AEs occurred in 93.3% of patients at 1.5mg/kg Q4W, with the most common being neutropenia (66.7%) and thrombocytopenia (46.7%).
– Infections were reported in 26.7% of patients at 1.5mg/kg Q4W (grade 3 in 16.7%).
– 22.6% of patients experienced infusion-related reactions
– The study did not observe the neuropsychiatric toxicities typically associated with interferon alfa.
Conclusions:
– Modakafusp alfa demonstrated single-agent activity and a manageable safety profile in heavily pretreated R/R MM patients.
– The immunocytokine targets interferon α to CD38+ cells, resulting in immune activation and antitumor activity.
– The study suggests a potential for combination with other anti-myeloma therapies due to its immune stimulatory effects, distinct CD38 binding, and manageable toxicity.”
Targeted interferon therapy with modakafusp alfa for relapsed or refractory multiple myeloma
Authors: Dan T. Vogl et al.



