Vincent Rajkumar shared on X:
“FDA approval doesn’t necessarily mean standard of care. For example, FDA approved Dara VMP for frontline therapy in myeloma in 2018.
Literally no one used the regimen in the US. Literally no one felt the regimen was standard of care in the US. Before or after approval! Why?
FDA adjudicates a sponsors submission on whether a given drug/regimen has met the burden of proving safety and efficacy.
Standard of care in clinical practice is a different standard: judgment of risk/benefit of available alternatives, and assessment of trial design/end points.
When Dara VMP was approved we had long back stopped using melphalan as frontline therapy in the US. So the regimen was really not relevant even though it was approved.
The type of trial design and proof needed for FDA (regulatory) Approval and setting Standard of Care may be similar or quite different depending on the disease and setting.
What’s enough for us may not be enough for FDA. What’s enough for the FDA may not be enough for us.
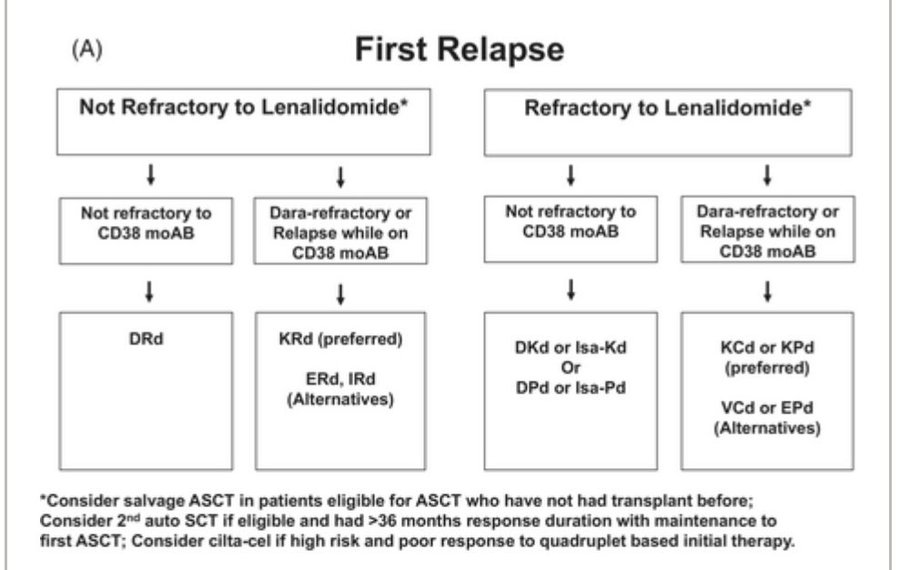
For example FDA has approved cilta-cel for first relapse in myeloma. But we do not even recommend it for treatment of first relapse except in rare settings, let alone consider it as standard of care.
That is because even though cilta-cel proved clinical benefit in the eye of the FDA in first relapse, oncologists make a judgment comparing it to other available treatments including risks, costs, efficacy, to make a decision on how and when to use it in the treatment of myeloma.
FDA typically looks at whether a given trial design is good, adequately powered, isolates the drug effect, and demonstrates adequate efficacy and safety over a Control group (randomized usually, or real world).
But sometimes regulators may be ok with endpoints and trial designs that the physician community may or may not feel is sufficient to change standard of care relative to other available options.
Also the magnitude of benefit that the FDA feels is sufficient to grant approval may or may not be sufficient for patients or physicians to change standard of care.
So sometimes weak drugs that squeak through by meeting the minimum requirements don’t even sway practice.
FDA approval is a key step. It serves an important purpose. Sometimes the approval changes the standard of care.
Sometimes it provides a new treatment option in a setting where no alternatives exist. Sometimes it doesn’t affect either.
One additional point. The FDA doesn’t get to adjudicate every treatment that we use in clinical practice in various disease settings.
They only adjudicate what is brought before them by a sponsor in the form of a proper regulatory submission. We have a lot of off label uses.
The initial FDA approval is the one that is critical for companies and companies understand this well.”
Source: Vincent Rajkumar/X
Vincent Rajkumar is a Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and Chair for the Mayo Clinic Myeloma, Amyloidosis, and Dysproteinemia Group.
He also chairs the Board of directors of The International Myeloma Foundation and the Eastern Cooperative Oncology Group (ECOG) Myeloma Committee.
His extensive contributions include over 230 peer-reviewed publications, predominantly focusing on multiple myeloma and related plasma cell disorders.
Furthermore, Dr. Rajkumar is a Section Editor for multiple myeloma and related disorders for Leukemia and an Associate Editor for the Mayo Clinic Proceedings.