Francisco Conesa Buendía, Assistant in Cell and Gene Therapies Manufacturing at Memorial Sloan Kettering Cancer Center, shared a post on LinkedIn:
“FAS-Less Allogeneic CAR T Cells: A Leap Towards Off-the-Shelf Cancer Therapies.
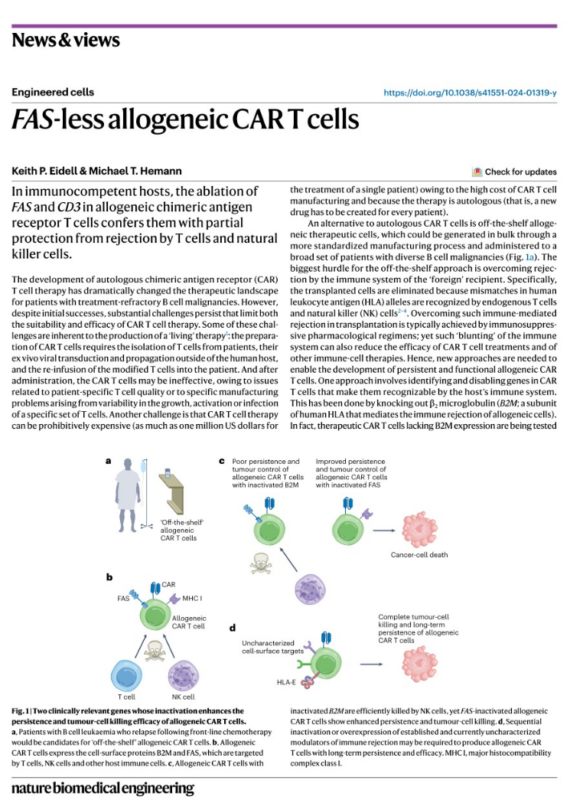
Recent advancements in CAR T cell therapies are offering hope to patients with treatment-refractory B-cell malignancies. This study published in Nature Biomedical Engineering, give us on a groundbreaking approach to improve the efficacy of allogeneic CAR T cells through FAS gene inactivation.
Key Findings:
Gene Inactivation Breakthrough:
Using a whole-genome CRISPR–Cas9 screen, the study identified FAS (Fas Cell Surface Death Receptor) and beta-2 microglobulin (B2M) as critical targets to improve persistence and immune evasion of CAR T cells in allogeneic settings.
FAS inactivation outperformed B2M, protecting CAR T cells from natural killer (NK) cell-mediated rejection while enhancing cytokine production and tumor cell killing in vitro.
- Enhanced Efficacy:
Functional assays with CD19-targeting CAR T cells revealed that FAS inactivation:
- Enhanced cytokine production.
- Upregulated activation markers like CD69, correlating with more effective leukemia cell killing.
- In mouse models of leukemia, FAS-deficient CAR T cells demonstrated:
- Delayed tumor growth and extended survival.
- Greater persistence compared to B2M-deficient CAR T cells.
Clinical Context:
In pre-relapse leukemia models (CAR T cells introduced before leukemia onset), FAS inactivation provided prolonged persistence, critical for sustained antitumor activity.
Why This Matters:
Current CAR T therapies face steep hurdles:
Cost: Patient-specific manufacturing raises therapy costs to nearly $0.5M- 1M per treatment.
Limitations of Autologous Approaches: Patient T cells may be unsuitable for manufacturing due to poor quality or variability in activation and growth.
Immune Rejection in Allogeneic Therapies: T and NK cells eliminate “foreign” cells, reducing efficacy.
FAS inactivation addresses these issues by enhancing the resilience and persistence of CAR T cells, paving the way for more effective off-the-shelf options.
Remaining Challenges:
Despite these advancements, hurdles remain:
- Moderate Efficacy: While promising, FAS inactivation alone doesn’t ensure complete protection from rejection.
- Safety Concerns: Potential for uncontrolled proliferation of CAR T cells raises safety and regulatory questions.
- Therapeutic Context: Results need validation in models that better mimic real-world leukemia treatment scenarios.
Future Directions:
This study highlights the need for combination strategies, such as simultaneous gene inactivation (e.g., FAS and other immune-modulating targets) or base editing approaches. By expanding genetic modifications, researchers aim to enhance persistence, tumor killing, and safety in allogeneic CAR T cells.
Francisco Conesa Buendía is an Assistant in Cell and Gene Therapies Manufacturing at Memorial Sloan Kettering Cancer Center, where he supports the genetic engineering and clinical-grade manufacturing of T lymphocytes (CAR-T) for phase I/II clinical trials. Prior to this role, he was a Postdoctoral Scientist at Fundación Progreso y Salud, specializing in the GMP manufacturing of Advanced Therapy Medicinal Products (ATMPs), including human MSCs and chondrocyte cultures.


