Dana-Farber’s Breast Oncology Center recently shared an article authored by Shadab A. Rahman and colleagues on X:
“This recent study looked at the incidence of patient-reported fatigue developing on palbociclib and Endocrine Therapy for advanced HR+ HER2- Breast Cancer, read it here.”
Authors: Shadab A Rahman et al.

This study showed that a common side effect of the CDK4/6 inhibitor palbociclib in patients with metastatic breast cancer (MBC) is fatigue, with rates of all-grade fatigue reported by clinicians at 39.2%, and 2.5% experiencing grade 3/4 fatigue. The aim was to evaluate the incidence of fatigue emerging during palbociclib treatment using patient-reported measures, as well as to explore potential factors that might predict its occurrence.
The study involved 88 patients with HR+ HER2- MBC who were starting palbociclib combined with endocrine therapy and had no baseline fatigue.
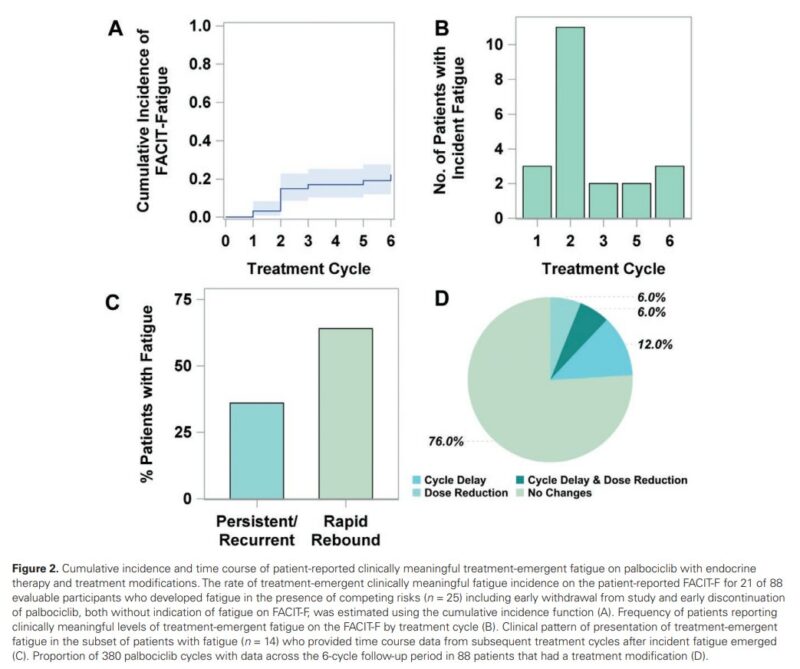
These patients were assessed before starting treatment and monthly over the first six cycles. Fatigue was evaluated using the Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-F), with scores below 34 indicating clinically meaningful fatigue, and the NCI Patient-Reported Outcomes for CTCAE (PRO-CTCAE) to measure the severity and functional impact of fatigue. Both hematologic and nonhematologic factors were analyzed to see if they could predict fatigue.
The results showed that 23.9% of patients reported clinically meaningful fatigue within an average of 2.8 ± 1.7 cycles of treatment. Severe fatigue, according to PRO-CTCAE ratings, occurred in 14.8% of patients, and 10.2% reported significant interference in their daily activities due to fatigue.
Lower pretreatment absolute neutrophil count (ANC) levels were linked to a higher likelihood of developing treatment-emergent fatigue, but ANC levels during treatment were not predictive. Other pretreatment factors, such as longer sleep duration and lower physical activity, were also found to be potential predictors, though the latter was only a trend. Additionally, patients who developed fatigue during treatment tended to have longer sleep durations, but other factors, including physical activity, did not show significant associations.
Nearly one-quarter of patients with HR+ HER2- MBC who begin palbociclib treatment report fatigue that develops quickly and can be severe, with a significant impact on daily functioning. Both pretreatment factors (like low ANC and long sleep duration) and factors that emerge during treatment (such as continued long sleep duration) are linked to the development of fatigue.