Oncology Brothers shared a post on X:
“SABCS24 Day 1 Highlights CommunityOnc:
- FDA Oncology Reviews recent approvals: Ribociclib & Inavolisib
- PADMA: HR+ ET + CDK4/6i vs Chemo 1L
- DB06: HR+ TDXd
- KN522: biomarker analysis”

“1. Recent U.S. FDA approvals:
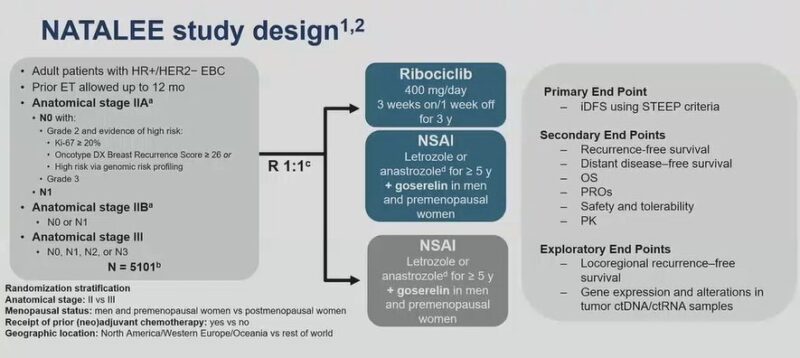
a). NATALEE: Adj Ribociclib in HR+ Stg IIA – III, 400mg 3wks on, 1wk off for 3yrs. 3yr iDFS 90.7% vs 87.6, 3.1% absolute iDFS
b). INAVO120: Inavolisib + Fulvestrant + Palbo vs Palbo/Fulvestrant HR+ mBC. PFS 15.0 vs 7.3mos (HR 0.43)”
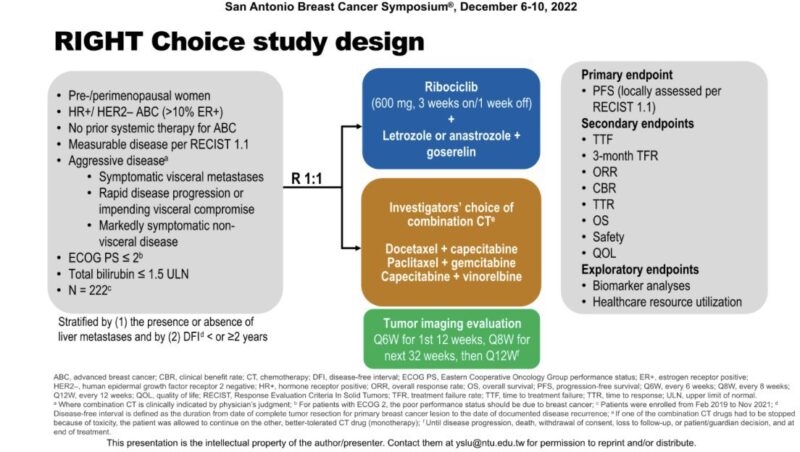
“2. PADMA: Ph III, HR+ metastatic breast cancer, chemo vs ET + Palbociclib.
– SABCS22 in Right Choice study, we knew CDK4/6i upfront is better.
– Time to treatment failure and PFS better in PADMA. CDK4/6i remain 1L SoC for these pts.”
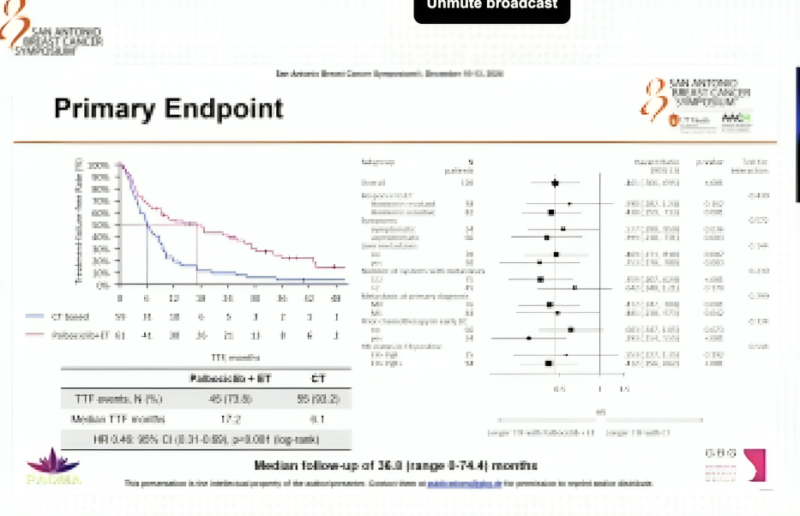
Quoting Sara Tolaney‘s post:
“PADMA study: ET + palbo vs chemo (cape/taxane/navelbine) in high risk HR+ MBC–> confirms ET+ cdk4/6i is 1L SOC
- n=150
- 42% w/liver mets
- 6% BRCAm
- TTF: 17.2 vs 6.1mo HR 0.46
- PFS: 18.7 vs 7.8 mo, HR 0.45
- OS trend: 46.1 vs 36.8 mo.”
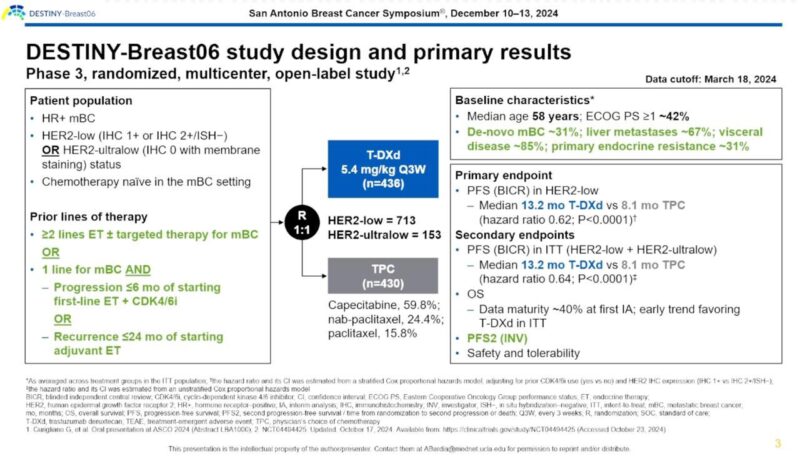
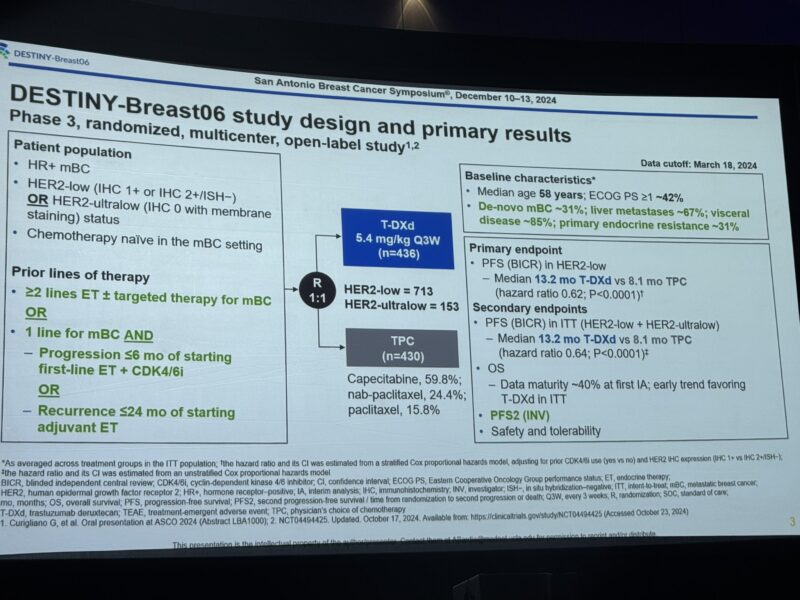
“3. DB06: . DestinyBreast06: Ph III, TDXd vs chemo in endocrine resistant HR+ HER2 low and HER2-ultralow
– Improved PFS regardless of time to progression on CDK4/6i or tumor burden
– PFS2 better w/ TDXd
– In what line will you use this.”
“Quoting Erika Hamilton‘s post:
- Story short, TDXd was better in all groups.
- Quick or slow relapsers on DB-06.
- Regardless of disease burden.
- Among primary or secondary endocrine resistance.”
“4. KN522: current SoC PeriOp IO + neoAdj Chemo and then Adj IO for high risk TNBC.
– This remains the current standard of care. As of now, there are no specific markers available in clinical practice to predict IO benefit in these patients.”
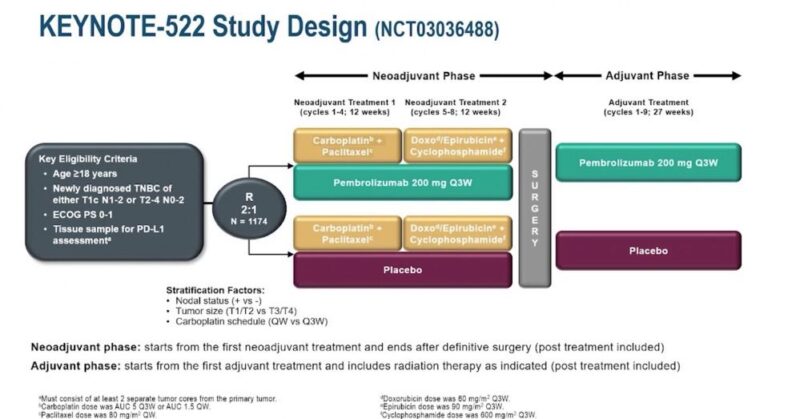
“Quoting Harold J. Burstein‘s post:
“Update on KN522 study.
Extensive biomarker analysis of subgroups of TNBC shows that…all benefit from pembro.
So far no selective marker for who does or does not need chemo +IO.””
Drs. Rohit Gosain and Rahul Gosain (aka Oncology Brothers), are medical oncologists/hematologists. Through their social media platforms like X/Twitter, they actively engage with their audience. With their own YouTube channel and podcast, they provide accessible resources for cancer patients and their families. Additionally, their participation in live conferences ensures that cancer patients receive the best care closer to home, reflecting their commitment to advancing oncology and improving patient outcomes through education and outreach.
Sara Tolaney is the Chief of the Division of Breast Oncology at Dana-Farber Cancer Institute. She also serves as Associate Director of the Susan F. Smith Center for Women’s Cancers and is a Senior Physician at Dana-Farber Cancer Institute and Associate Professor of Medicine at Harvard Medical School. Her research focuses on the development of novel therapies in the treatment of breast cancer and developing more effective and less toxic treatment approaches. Her work has demonstrated that a relatively low risk regimen is beneficial in women with early stage node-negative HER2-positive cancers, and this works has been incorporated into national and international guidelines. She has developed several follow-up studies looking at novel approaches to early stage HER2-positive disease and has also played a significant role in development of cdk 4/6 inhibitors, antibody drug conjugates, and immunotherapy in breast cancer. She is the author of over 150 peer-reviewed publications in leading scientific journals. She is the recipient of the Lee M Nadler “Extra Mile” Award, and the Innovation Award for Clinical Faculty.
