Vincent Rajkumar, Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, posted on X about recent paper by him as co-author, titled “Daratumumab or Active Monitoring for High-Risk Smoldering Multiple Myeloma” published on The New England Journal of Medicine.
Authors: Meletios A. Dimopoulos, Peter M. Voorhees, Fredrik Schjesvold, Yael C. Cohen, Vania Hungria, Irwindeep Sandhu, Jindriska Lindsay, Ross I. Baker, Kenshi Suzuki, Hiroshi Kosugi, Mark-David Levin, Meral Beksac, Keith Stockerl-Goldstein, Albert Oriol, Gabor Mikala, Gonzalo Garate, Koen Theunissen, Ivan Spicka, Anne K. Mylin, Sara Bringhen, Katarina Uttervall, Bartosz Pula, Eva Medvedova, Andrew J. Cowan, Philippe Moreau, Maria-Victoria Mateos, Hartmut Goldschmidt, Tahamtan Ahmadi, Linlin Sha, Annelore Cortoos, Eva G. Katz, Els Rousseau, Liang Li, Robyn M. Dennis, Robin Carson, S. Vincent Rajkumar.

“Just out: Paradigm changing AQUILA randomized trial in high risk smoldering myeloma.
Daratumumab significantly prolongs time to active myeloma and overall survival. Proud to be a lead investigator of this trial.
Main Findings Daratumumab prolongs:
-Time to active myeloma
-Time to CRAB
-PFS2
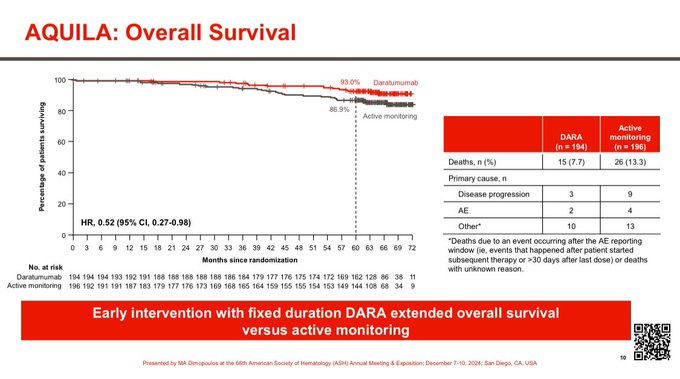
-Overall Survival
Plus: Low toxicity, no detriment to QOL, and limited duration therapy.
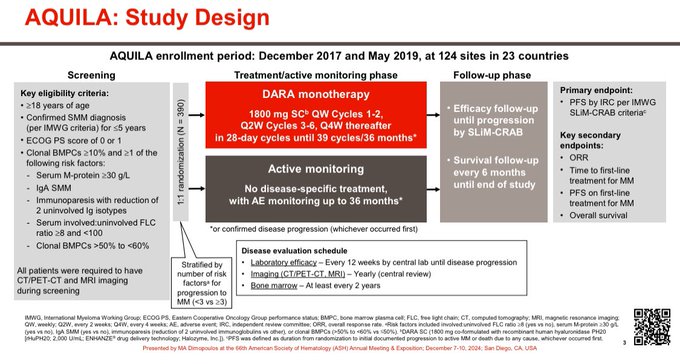
390 patients with high risk SMM randomized. Largest trial in SMM ever conducted.
Limited duration therapy.
Significant improvement in time to progression to active myeloma or death with Dara. Benefit seen in almost all subgroups.
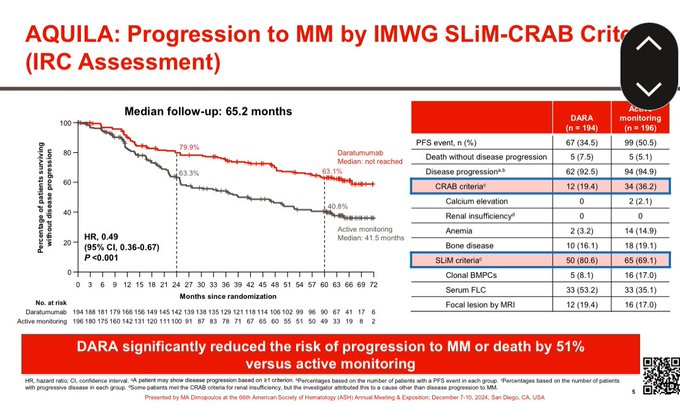
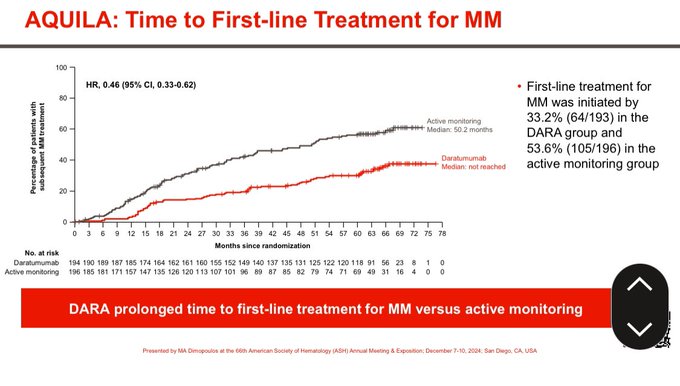
64% single agent response rate with Dara. Significant prolongation of time to needing active myeloma therapy.
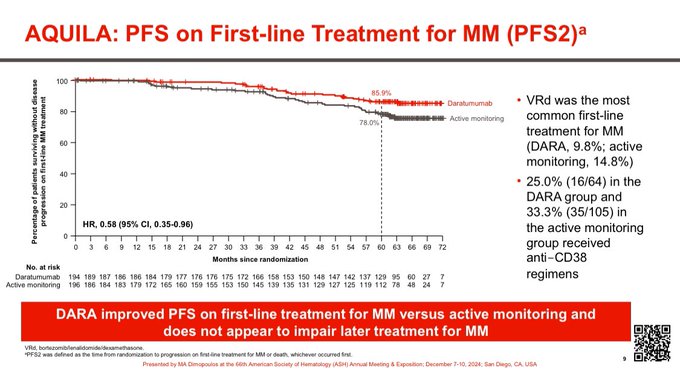
Improved PFS2 No adverse impact of Dara therapy on subsequent outcomes.
Superior Overall Survival with Dara. HR 0.52 95% CI 0.27-0.98
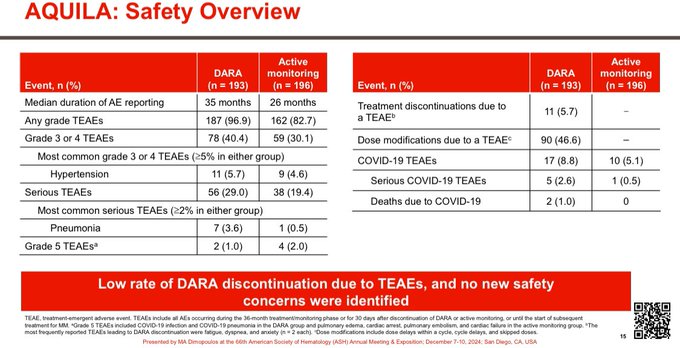
Good safety profile. Similar numbers despite longer follow up.
There is a 10% absolute higher risk of grade 3-4 AEs some of which is related to longer period on study in Dara arm.
No detriment on quality of life measurements.
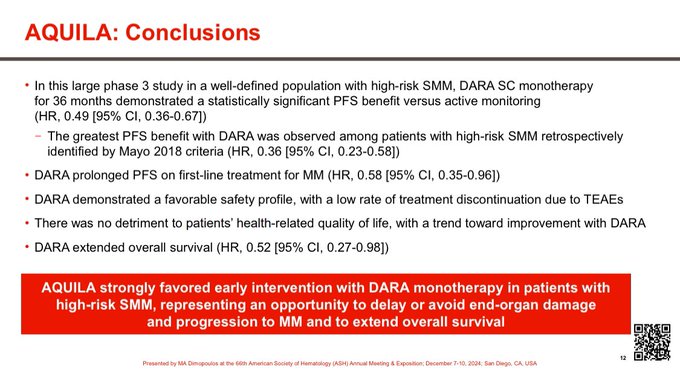
Conclusions.
In high risk smoldering myeloma,
Daratumumab prolongs:
-Time to active myeloma
-Time to CRAB
-PFS2
-Overall Survival
Low toxicity, no detriment to QOL, and limited duration therapy.
Vincent Rajkumar is a Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and Chair for the Mayo Clinic Myeloma, Amyloidosis, and Dysproteinemia Group. He also chairs the Board of directors of The International Myeloma Foundation and the Eastern Cooperative Oncology Group (ECOG) Myeloma Committee. His extensive contributions include over 230 peer-reviewed publications, predominantly focusing on multiple myeloma and related plasma cell disorders. Furthermore, Dr. Rajkumar is a Section Editor for multiple myeloma and related disorders for Leukemia and an Associate Editor for the Mayo Clinic Proceedings.


