Nina Niu Sanford, Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital, shared an article on X:
“Our comprehensive assessment of NCI cooperative group trials is out in JNCI!
If you are involved or enrolling in coop trials, please consider reading ur major findings.”

Benchmarks of Success in Radiotherapy versus Systemic Therapy National Clinical Trials Network (NCTN) Randomized Controlled Trials Sponsored by the National Cancer Institute (NCI).
Authors: Nina N Sanford, et al.

“First, this was an extension of a smaller project last year looking at endpoints & patient selection criteria in cooperative group trials assessing radiotherapy for GI cancers.
We decided to expand to other disease sites & do additional analyses.”
Elucidating the Benefit of Radiation Therapy in GI Cancers: Rethinking Trial End Points and Patient Selection.
Authors: Nina N. Sanford, et al.

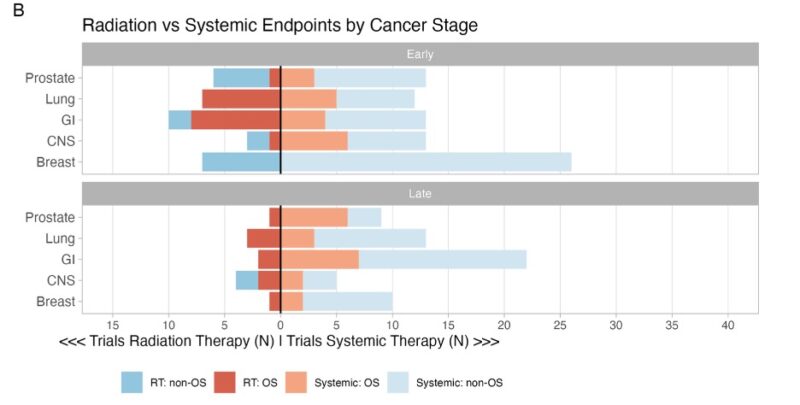
“For this study, we compared study design in radiation versus systemic cooperative group trials across 5 majors disease sites: breast, prostate, CNS, GI and lung – the sites with greatest number of RT trials.
Our study included a total of 186 RCTs.”
“First, primary endpoint. Across sites, 59% of radiation trials had primary endpoint of OS versus 27% of systemic trials.
There were differences by disease site though. For example, in lung cancer, 100% of RT trials use OS (vs 30% systemic).”
“We also stratified by Stage and trial Phase. Even for Stage 4 cancer, 100% of RT trials in prostate/breast/lung/GI had primary endpoint OS vs lower in systemic (20% breast, 23% lung, 32% GI, prostate was 67%).”
“For trials with non-OS primary endpoint, we categorized the type of endpoint selected.
Most were composite surrogate endpoints (PFS, DFS, EFS) rather than symptom-based or QOL. See paper for deets.”
“Second, superiority vs. non-inferiority trials. A total of 31% (!) of RT trials were non-inferiority studies assessing radiation omission or de-escalation.
This is in contrast to 6.3% of systemic studies.”
“In breast cancer, 75% of radiation studies were omission trials!
The raw of RT trials (even in coop group) is much less than systemic…and most of them here are looking at ways to eliminate RT.”
“We also studied alpha, power and effect size, which were broadly similar by modality, although there were outliers.
We provide ranges for these measures, by trial Phase, that can be considered in future studies.
Much more other stuff in paper.”
“A final point.
These basic features on statistical design were often hard to find, and some protocols didn’t include things like alpha anywhere.
This info should be presented in a structured format near front, not buried in text at the end of these long protocols.”
“Huge thanks to my mentor Bill Hall, ALLIANCE statistician Qian Shi & my former research assistant David Hein for collaborating on this project!”
“Lastly, on a personal note, I’ve really enjoyed working with coop groups/NRG Oncology.
They are arguably the most important mechanism for conducting RT trials in the US.
So that’s why I think it’s super important to assess the status of RT trials & brainstorm ways to do better.”
Nina Niu Sanford is an Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital. She specializes in treating gastrointestinal cancers and actively participates in clinical trials combining high-dose radiation therapy with immunotherapy. Additionally, she researches healthcare access disparities and conducts pan-cancer outcomes research using large databases.


