Tejas Patil, Thoracic oncologist at University of Colorado Cancer Center, posted on X about recent paper by him as first author, titled “The efficacy of continuing osimertinib with platinum pemetrexed chemotherapy upon progression in patients with metastatic non-small cell lung cancer harboring sensitizing EGFR mutations” published on Lung Cancer Journal.
Authors: Tejas Patil, Dexiang Gao, Alexander Watson, Mandy Sakamoto, Yunan Nie, Amanda Gibson, Michelle L. Dean, Benjamin A. Yoder, Eliza Miller, Margaret Stalker, Dara L. Aisner, Paul A. Bunn, Erin L. Schenk, Melina E. Marmarelis, Chiara Bennati, Vishal Navani, Yongchang Zhang, D. Ross Camidge.
“In patients with EGFR, NSCLC, what is the value of continuing osimertinib with platinum-pemetrexed after progression vs stopping osimertinib and switching to platinum pemetrexed? I’m proud to present our multi-center, retrospective, international series published in Lung Cancer Journal.
Despite improved understanding of osimertinib resistance mechanisms, ~45% patients with EGFR, NSCLC have no mechanism of resistance identified on progression. Prior to MARIPOSA-2, platinum-pemetrexed (without osimertinib) was routinely recommended with mPFS ~4 mos.
Our international retrospective study included patients with EGFR, NSCLC who progressed on osi (n = 159) and received next line platinum-pemetrexed. Primary endpoints = PFS and OS. Exclusion = stage I-IIIB, atypical EGFR mutations, nonplatinum pemetrexed doublets, incomplete follow up.
STATS + SCHEMA: Unadjusted survival curves done via Kaplan-Meier methods. Multivariable adjustment for prior lines of therapy, brain mets, oligometastatic disease (defined as ≤ 3 metastatic sites), TP53 mutations, bevacizumab use, and immunotherapy use.
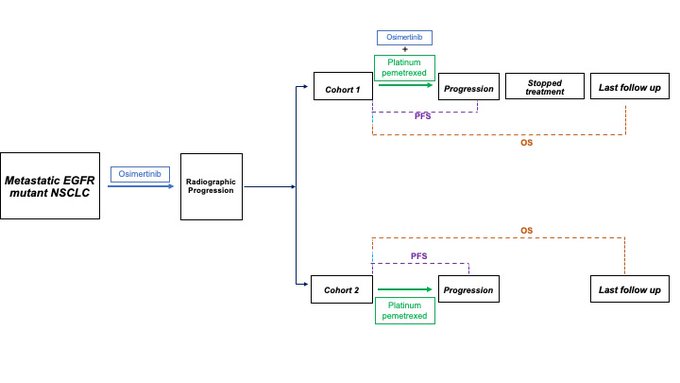
POINT 1: Continuing osimertinib wuth next line platinum pemetrexed associated with improved PFS vs platinum pem alone (9.0 vs 4.5 months; HR 0.49, 95 % CI 0.32 – 0.74, p = 0.0032). Our control arm had mPFS of 4.1 mos; approximates the control arm of the MARIPOSA-2 (mPFS 4.2 mos)
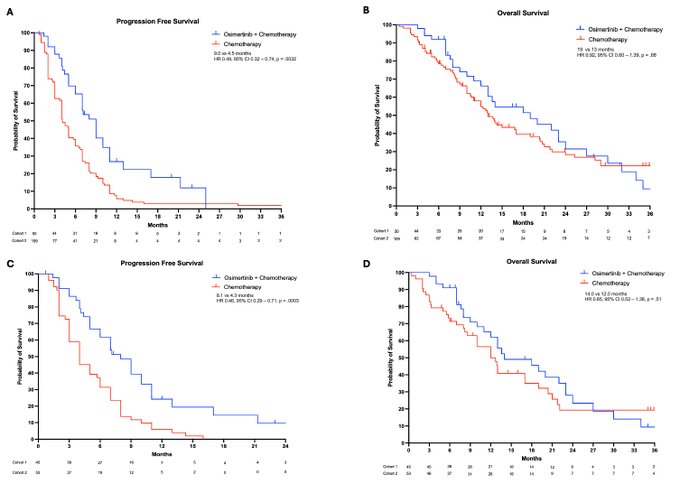
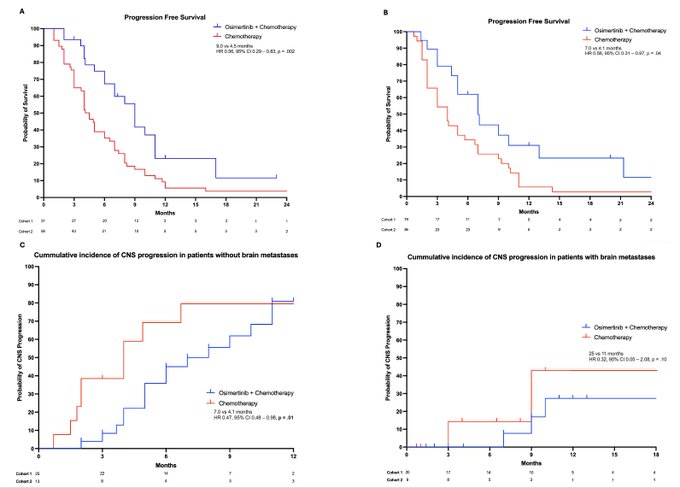
POINT 2: Improved PFS may be related to reduction in CNS metastases. In patients with EGFR mutant NSCLC WITHOUT brain mets after progression on osimertinib, continuing osimertinib with platinum pem improved the median time to CNS progression (n = 38; 7.0 vs 4.1 months; HR 0.47; Fig 3C)
POINT 3: There was numeric, but not significant OS difference with continuing osi with next line platinum-pemetrexed. Multivariable modeling suggests that bevacizumab + platinum pemetrexed associated with a 44 % lower risk of death (HR 0.56, 95 % CI 0.33-0.90, p = 0.03)
LIMITATIONS: 1) Retrospective analysis, so association does not equal correlation; 2) Variability in CNS imaging (can’t get CNS ORR or CNS PFS); 3) Limited post-osi molecular profiling hard to know whether certain resistances (eg, C797S, HER2 amp) benefit from osi+platinum-pem
Ongoing COMPEL trial (NCT04765059) will answer this question prospectively. Currently, MARIPOSA2 (ami+chemo) SOC, though our retrospective study adds to literature by showing relevance of EGFR inhibition (either with osimertinib OR amivantamab) with next line platinum pemetrexed.”


