Natasha Rekhtman posted on X:
“Hot off the press from team Memorial Sloan Kettering Cancer Center in Cancer Discovery! A study solving the puzzle of exceptional new lung cancer type: Chromothripsis-mediated Small Cell Lung Cancer SCLC This took nearly a decade and 42 coauthors to unravel. Here are some highlights.
Nearly all SCLC arise in smokers and have RB1 and TP53 mutations. Over the years, we encountered exceptional patients breaking all the rules: pathology of SCLC, but with intact RB1 and TP53, and generally never/light smokers. These are rare (3% of SCLC), but solving them was an opportunity to uncover unique biology and to inform diagnosis and treatment for when these patients are encountered in practice.
So what drives these unusual tumors? Remarkably, we found that in nearly all cases it is massive chromothripsis: ‘chromo’ – chromosome, ‘thripsis – shattering. A process where in single catastrophic event, one or several chromosomes shatter and realign in “Frankenstein-like” ways. Entirely different from the well-established concept of gradual mutation accumulation.
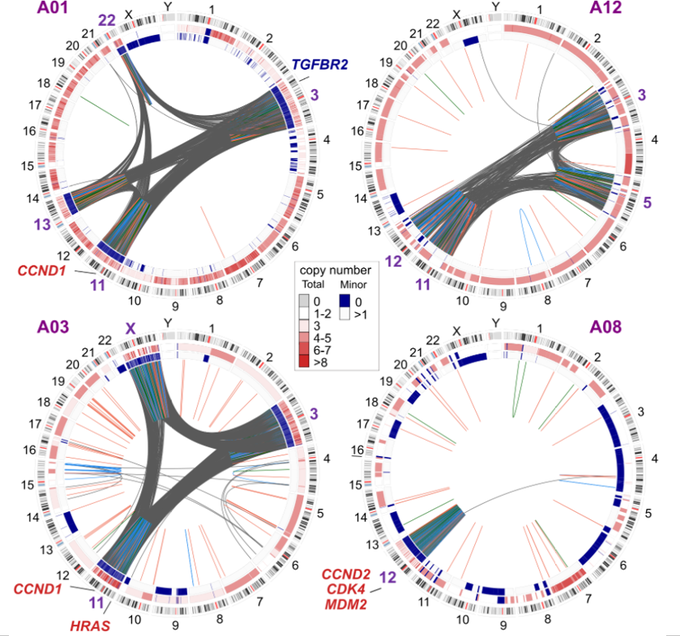
Here are the so-called ‘circos plots’ from whole-genome sequencing showing absolutely massive number of rearrangements between the shattered chromosomes in our cases, reaching ~2K per case!
Another major consequence of chromothripsis if that DNA realigns as extra-chromosomal (ec) circular DNA (aka double-minutes), where oncogenes can get amplified to colossal copy numbers. And this is what we found happens in these tumors. They have recurrent massive ecDNA amplification of several oncogenes, most notably CCND1, CDK4 and MDM2, which then functionally inhibit pRb and p53. All roads lead to Rome!
Here is a striking example of FISH showing ecDNA amplification of CCND2, MDM2 and CDK4 in a case with chromothripsis of chromosome 12, which houses these genes.
The other major finding is the link between these chromothripsis-associated SCLC and … lung carcinoids – the lower-grade and generally indolent neuroendocrine tumors. Textbook concept is that carcinoids DO NOT progress to SCLC. These exceptional cases overturn this dogma. Here is an example where pathologically there was carcinoid histology in thoracic tumor and classic SCLC in the brain.
We took a deep dive to characterize pathologic, radiologic, germline and clinical features associated with these unique tumors. Please see our paper for details. For pathologists, we included extensive additional illustrations of path findings in the supplement – please check it out!
Lastly, the other major analysis in this paper was to compare chromothripsis-associated SCLC with another rare subtype of SCLC… that arises in never-smokers but WITH RB1/TP53 mutations. This group is better characterized, but this study included one of the largest cohorts of this type. They contrast sharply with chromothripsis-mediated SCLC, and suggest 2 pathways for how SCLC arise in never smokers:
Thinking nearly a decade back, when Charlie Rudin and I started to encounter these patients (Charlie in the clinic and I in through the microscope), we knew we were dealing with a unique new tumor type, but it took a long journey together with an amazing team MSK to actually solve this incredible puzzle. Although much work remains to find best tailored treatments for these tumors – the study paves the way.
I wanted to give a major shoutout to my co-PI Charles Rudin for an incredibly synergistic collaboration, and my co-first authors who worked tireless on this story Sam T and Chris Febres. Thank you Jake June-Koo Lee and Benjamin Herzberg for your essential genomic and clinical contributions this study. And thanks to the entire team Memorial Sloan Kettering Cancer Center for a phenomenal team effort.”
Source: Natasha Rekhtman/X
Natasha Rekhtman is a physician at Memorial Sloan Kettering Cancer Center, where she specializes in diagnostic pathology. Her expertise includes thoracic pathology and cytopathology, focusing on the accurate diagnosis and management of diseases affecting the lungs and other thoracic organs, as well as cellular abnormalities.