
Felipe Rodrigues: Excited to share our work on the reciprocal interaction between metastatic cancer cells and AT2 cells
Felipe Rodrigues, Postdoctoral Researcher at Rockefeller University, recently shared a post on X:
“Beyond excited to share our work on the reciprocal interaction between metastatic cancer cells and AT2 cells now published at Developmental Cell.
We employed an in vivo labeling tool previously developed in our lab by the incredible Luigi Ombrato, now group leader of Barts Cancer Institute (Queen Mary) (QMBCI). More details on the labeling tool below.
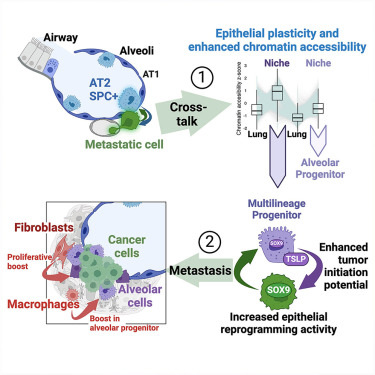
By focusing on breast cancer metastases to the lung, we found that alveolar type 2 (AT2) cells within the niche exhibited a program of lineage infidelity. Instead of generating their typical alveolar organoid, some niche AT2 cells were now making organoids of other lung lineages.
With help from the fierce Adam Karoutas, we’ve shown that the activation of this multilineage stem-like state was accompanied by significant chromatin remodeling, with niche AT2 cells having higher chromatin accessibility than their distal counterparts.
We combined a series of lung organoid/tumouroid cultures with in vivo assays to demonstrate that cancer cells reprogram AT2 cells by physical interaction.
But that’s not all – our data suggest that metastatic cells benefit from adapting to the alveolar environment, and consequently gain the ability to reprogram AT2 cells via paracrine factors.
We then found that metastatic breast cancer cells up-regulate Sox9 expression upon interaction with the alveolar environment, which promoted their metastasis-initiating potential while simultaneously also increasing Sox9 expression in AT2 cells.
Interestingly, not only nuclear Sox9 is higher in metastases than primary tumors, but cancer cells isolated from the lungs exhibit a much better capacity to reprogram AT2 cells than cancer cells isolated from primary tumors – Thanks Rev3 for suggesting this experiment!
What happens then if we target Sox9 expression in cancer cells? Their AT2 cell neighbors now lose their multilineage differentiation ability!
Finally, by performing co-culture experiments of AT2 cells and cancer cells alongside tumourigenic assays in vivo, we showed that AT2 cells support the stemness and tumor-initiating ability of cancer cells.
Please check our paper for more details. We also touched on fibroblasts and macrophages within the metastatic niche and their potential to influence AT2 cell behavior ex vivo.
Altogether, we believe our findings unearthed a new conceptual framework during metastatic niche initiation that we named ‘reflected stemness’.
In this model of reciprocal interaction, metastatic cells reprogram the local tissue into a stem-like state that enhances intrinsic cancer-initiating potential, thus creating a positive feedback loop where tumourigenic programs are amplified.
This work would not have been possible without all the support and mentorship I had from Ilaria Malanchi – it has been truly a privilege to work in her lab The Crick.
Huge thanks to my friends and colleagues for their immense support and contributions.”
Read further
Source: Felipe Rodrigues/X
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
