
Apr 8, 2024, 16:01
Rahul Gosain: Trastuzumab Deruxtecan now is FDA approved, Based on DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02
Rahul Gosain, Co-Founder of Oncology Brothers podcast and Director of Regional Infusion Services and Medical Director at Wilmot Cancer Institute, recently shared on LinkedIn:
“Trastuzumab Deruxtecan now FDA approved for pan-tumor with HER2 IHC3 and solid tumors who have received prior systemic treatment and have no alternative treatment options.
Based on DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02:
- Dose 5.4 mg/kg
- Common AE: fatigue, decreased counts and nausea. ILD can be fatal.”
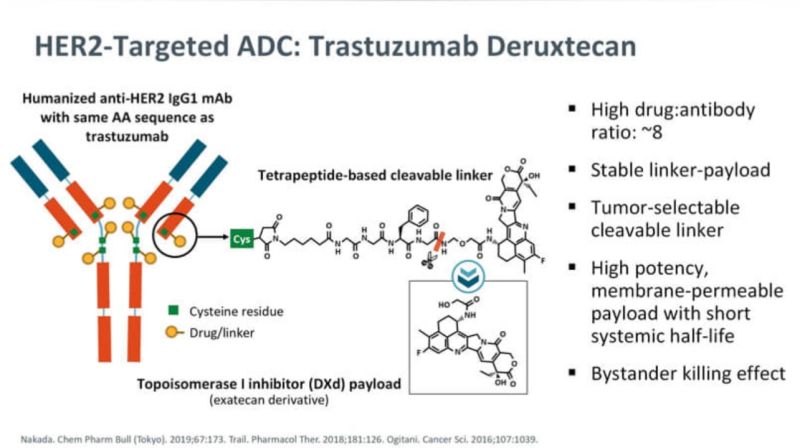
Source: Rahul Gosain/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Aug 21, 2025, 06:29
Aug 21, 2025, 00:00
Aug 20, 2025, 23:11
Aug 20, 2025, 21:51
Aug 20, 2025, 21:35
