Tejas Patil: A Multi-Center Phase 2 Trial of EV in Patients with Advanced NSCLC
Tejas Patil, Thoracic Oncologist at University of Colorado Cancer Center, shared on X:
“I’m thrilled to share the results from EV-202, a multi-center Phase 2 trial of enfortumab vedotin (EV) in patients with advanced NSCLC – now in Eur J Cancer – EORTC!
See below for highlights!
Title: Enfortumab vedotin in patients with advanced non-small cell lung cancer after disease progression on platinum- and PD-1/PD-L1 inhibitor-containing regimens: Phase 2 international multicenter EV-202 study
Authors: Kei Muroa, Trevor Feinstein, Joaquina Barandac, Ioana Bontad, Noriko Yanagitanie, Todd Gerstenf, Leena Gandhig, Toshihiro Kudoh, Naomi Fujiokai, Jason Kaplanj, Seema Gorlaj, Shubin Liuj, Michele Wozniakj, Srinivasu Poondruj, Ryan Dillonj, Changting Mengk, Tejas Patil
Nectin-4 is minimally expressed in healthy adult tissues, but is HIGHLY enriched in embryonic, placental, and cancer cell surfaces. Exact role in cancer is unclear, but appears to be involved in PI3K/AKT pathway, angiogenesis, and cell migration.
This is a NICE review of Nectin-4 biology!
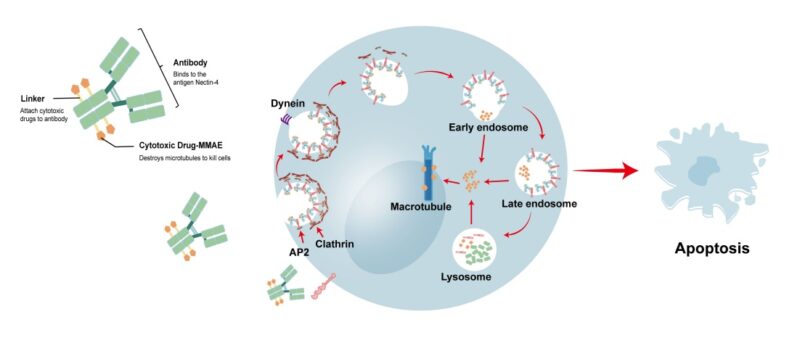
Enfortumab vedotin (EV) is an ADC that targets Nectin-4 with an MMAE payload. It is most well studied in urothelial carcinoma. This Phase 2 study explored the efficacy of EV in patients with NSCLC (cohorts 3 & 4).
Schema of study shown below.
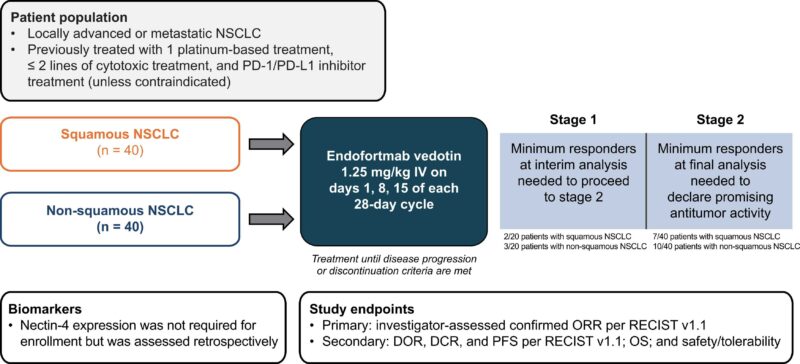
Total of 68 patients enrolled
- Squamous NSCLC cohort (n = 23)
- Non-squamous NSCLC cohort (n = 45)
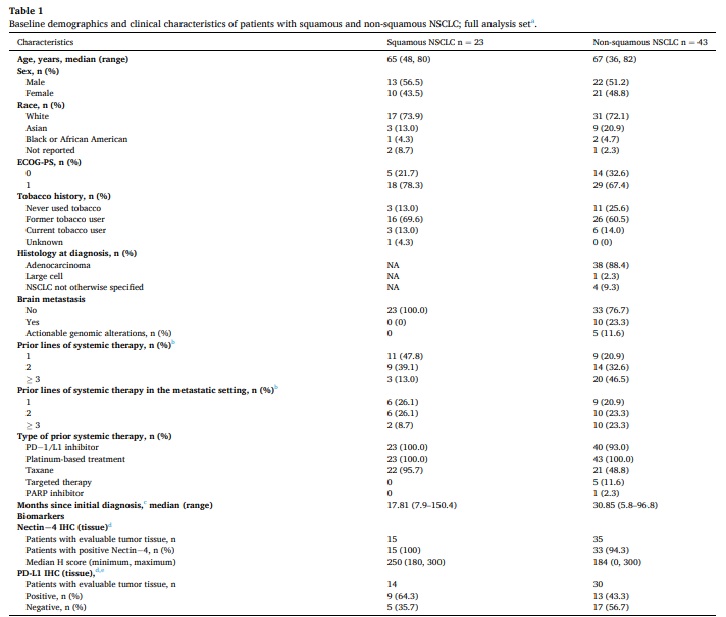
Table 1: Baseline characteristics shown below.
Some key points
- Nectin-4 expression was very high in both squamous & non-squamous patients.
- High percentage of patients in both cohorts with PDL1 < 1%
Efficacy (Part 1)
- Squamous cohort: ORR 4.3 % (95 % CI, 0.1–22.0 %). This did not meet minimum efficacy to continue further enrollment.
- Non-squamous cohort: ORR 14 % (95 % CI, 5.3–27.9 %), PR in 6 patients (14.0 %), median time to response was 1.8 months (range, 1.6–4.1), median DOR was 10.0 months (95 % CI, 9.7–10.4), DCR of 67.4 %. Didn’t meet minimum efficacy threshold either.
Key point: No correlation between Nectin-4 expression and EV response (Supplemental Figure 2; not shown)
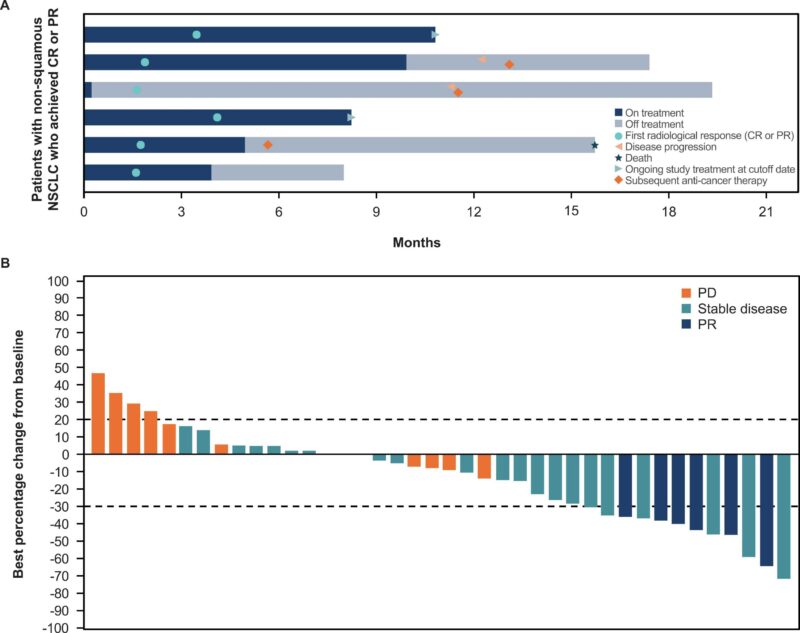
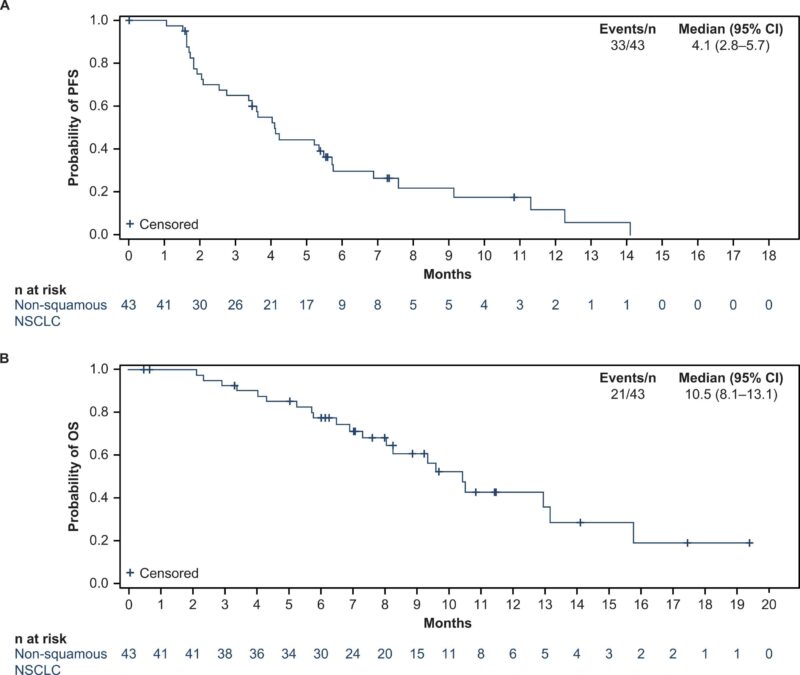
Efficacy (Part 2) – nonsquamous cohort only
- Median follow up 9.7 months
- mPFS – 4.1 months (95 % CI, 2.8–5.7)
- OS – 10.5 months (95 % CI, 8.1–13.1)
Safety
- Most common TRAEs (any grade) in ≥ 20 % of patients: rash (37.2%), neuropathy (37.2%), alopecia (32.6%), taste changes (27.9 %); nausea and fatigue (each 25.6 %); and diarrhea (23.3 %)
- Grade ≥ 3 TRAEs (13/43; 30.2 %): rash & neutropenia most common
- TRAEs led to dose reduction in 7 patients, dose interruption in 16 patients, & withdrawal of treatment in 4 patients.
Key point: AEs consistent with known profile of EV (i.e. neuropathy, skin rash, keratitis, neutropenia). Pneumonitis low in non-squamous population (2.3%)
Take home points
- Despite efficacy in urothelial carcinoma, EV did not meet primary efficacy endpoints in both squamous and non-squamous NSCLC.
- While responses were seen, no correlation between Nectin-4 IHC expression & tumor response.
- Safety signals consistent with EV in other studies
- Negative trials should be highlighted more often & do have value for advancing knowledge base in NSCLC!!
Congrats to ALL investigators, the sponsor, and most importantly, patients who participated!
Efficacy outcomes (Part 2).”
More posts featuring Tejas Patil.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023