
REGOMUNE Trial Comprehensive Overview in Advanced Solid Tumors
REGOMUNE Trial — a multicenter, prospective, open-label phase Ib/II study — explores the safety and effectiveness of combining regorafenib with avelumab in patients with advanced or metastatic solid tumors. Using a traditional 3+3 dose escalation design, the trial aims to determine the recommended phase II dose (RP2D) of regorafenib when paired with avelumab. Once established, the RP2D is tested across 17 cancer cohorts in the phase II stage. Additionally, a low-dose regimen of regorafenib (80 mg/day) with avelumab is being evaluated specifically in colorectal cancer (non-MSI-H/MMR-deficient) and other advanced cancer types.
Trial Design and Cohort Overview for Regorafenib–Avelumab Combination
-
Phase I (Dose escalation): Digestive solid tumors to define RP2D of regorafenib plus standard-dose avelumab.
-
Phase II: RP2D evaluated across cohorts including colorectal (non‑MSI‑H), GIST, GI cancers, biliary tract, HCC, sarcoma, thyroid cancer, GEP-NETs, NSCLC, and tumors with tertiary lymphoid structures (TLS+).
-
Low-dose Regorafenib + Avelumab (80 mg/day): Tested in colorectal cancer, urothelial carcinoma, HPV-associated tumors, triple‑negative breast cancer, TMB-high tumors, MSI-high tumors, non–clear-cell renal carcinoma, and mesothelioma.
Eligibility Criteria – Inclusion
The trial enrolls adult patients (≥18 years) with advanced, unresectable, or metastatic solid tumors across multiple cohorts, each defined by tumor type and molecular characteristics. Eligible cancers include non–MSI-H or dMMR colorectal cancer, gastrointestinal stromal tumor (GIST), esophageal or gastric carcinoma, hepatobiliary cancers, soft-tissue sarcoma (STS), radioiodine-refractory differentiated thyroid cancer (RR-DTC), neuroendocrine gastroenteropancreatic tumors (grade 2–3), non-small cell lung cancer (NSCLC), urothelial cancer, HPV-associated cancer, triple-negative breast cancer, TMB-high solid tumors, MSI-high solid tumors, non–clear-cell renal carcinoma, and malignant pleural mesothelioma. Certain cohorts require central histologic confirmation per INCa and RRePS guidelines.
Patients must have measurable disease by RECIST v1.1, ECOG performance status 0–1, and life expectancy >3 months. They should have received at least one prior systemic therapy (except in specified cohorts) or have no remaining established treatment options. Disease progression before study entry must be documented radiologically; in selected cohorts, this requires central review of sequential imaging.
Key laboratory requirements include adequate hematologic, renal, hepatic, and metabolic function (e.g., hemoglobin ≥9 g/dL, ANC ≥1.5 x 10⁹/L, platelets ≥100 x 10⁹/L, creatinine clearance ≥30 mL/min, liver enzymes ≤2.5 x ULN [≤5x ULN in certain metastatic settings]). Patients with hepatocellular carcinoma must have Child-Pugh A liver function.
At least 3 weeks must have elapsed since prior chemotherapy, immunotherapy, or radiotherapy, and any treatment-related toxicities should have resolved to grade ≤1 (except alopecia and non-painful neuropathy grade ≤2). No other malignancy should have been diagnosed or treated in the past 2 years, except certain in situ cancers and adequately treated skin cancers.
Women of childbearing potential require a negative pregnancy test within 72 hours prior to first dose, and all patients must agree to use highly effective contraception during treatment and for 8 weeks afterward. Informed consent, social security coverage per French law, and compliance with all cohort-specific prior treatment rules (e.g., prior PD-(L)1 therapy limitations, molecular status requirements) are mandatory.
Outcomes Measured
In this study, the phase I component focuses on determining the recommended phase II dose (RP2D) of regorafenib when combined with avelumab. Safety is closely monitored through the evaluation of dose-limiting toxicities and adverse events graded according to NCI CTCAE v5.0 criteria. Pharmacokinetic parameters, including area under the curve (AUC), half-life, and peak plasma concentration (Cₘₐₓ), are assessed to understand the drug’s behavior in the body. Antitumor activity is measured through the objective response rate (ORR), best overall response, one-year progression-free survival (PFS), and one-year overall survival (OS). Additionally, predictive biomarkers are explored through blood and tissue analyses to identify potential indicators of treatment response.
The phase II portion evaluates the efficacy of the regorafenib–avelumab combination across multiple tumor-specific cohorts. Outcomes include objective response rates, progression-free survival, and overall survival, with biomarker studies conducted in parallel. Depending on the cohort, these endpoints are measured over either six months or one year, allowing for a comprehensive assessment of both short-term and longer-term therapeutic benefits.
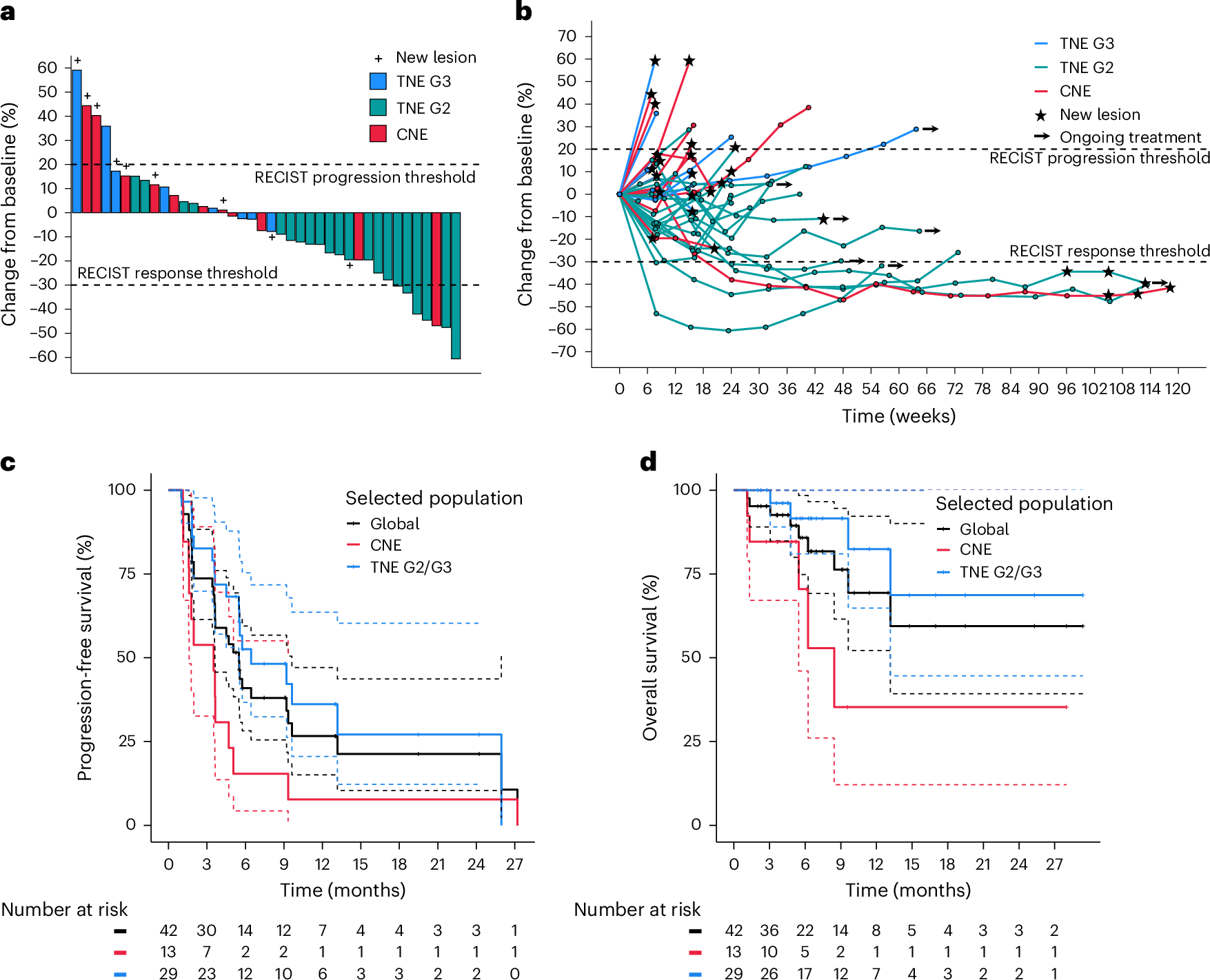
Efficacy and Biomarker Findings from the REGOMUNE Trial in MSS Metastatic Colorectal Cancer
Efficacy
- No objective responses (i.e., no complete or partial responses) were observed among the 43 efficacy-evaluable patients (6‑month ORR = 0%)
- Stable disease was achieved in 23 patients (53.5%).
- Progressive disease occurred in 17 patients (39.5%)
- Median PFS: 3.6 months (95% CI: 1.8–5.4)
- Median OS: 10.8 months (95% CI: 5.9–not reached)
-
Safety: Most common grade 3–4 adverse events included palmar‑plantar erythrodysesthesia (30%), hypertension (23%), and diarrhea (13%)
Biomarker Insights
- Patients with high baseline CD163+ tumor-associated macrophage infiltration had significantly worse outcomes (PFS: 1.8 vs. 3.7 months; OS: 3.7 months vs. not reached; P = 0.002)
- Increased CD8+ T-cell infiltration at Cycle 2 Day 1 (versus baseline) was associated with improved PFS and OS
Key Takeaways
-
The REGOMUNE trial did not meet its primary endpoint of objective responses in MSS colorectal cancer.
-
However, the treatment led to disease stabilization in over half of patients, and modest improvements in PFS and OS compared to historical regorafenib monotherapy.
-
Biomarker analyses revealed that the immune microenvironment—particularly macrophage and CD8+ T-cell infiltration—may influence outcomes, offering clues for future patient selection or therapeutic strategies.
About Institut Bergonié and Key Investigators
Institut Bergonié was founded in 1923 and named after the pioneering oncologist Jean-Alban Bergonié, Institut Bergonié is a premier regional cancer center in Bordeaux. Designated as a Cancer Research–Care (SIRIC) center, it operates under the “Curie model,” which emphasizes a seamless continuum from fundamental research to patient care With over 1,000 dedicated professionals, it serves as a leading hub for oncology treatment, training, and research across southwestern France
Prof. Antoine Italiano
Prof. Italiano leads the Early Phase Trials and Sarcoma Unit at Institut Bergonié. A medical oncologist with a PhD, he trained in both France and the U.S., including a postdoctoral fellowship at Memorial Sloan Kettering Since 2016, he has served as a Professor at the University of Bordeaux and spearheads the INSERM U1312 SARCOTARGET team, which focuses on the biology and therapeutic targeting of sarcomas
He plays a pivotal role in clinical research: over the past five years alone, he’s led more than 40 Phase I and over 20 Phase II/III oncology trials. He coordinates the national MULTISARC sequencing trial and the France 2030–funded CONDOR project, which leverages immunotherapy and AI for sarcoma treatment optimization A prolific researcher, Prof. Italiano is consistently ranked among the world’s most-cited scientists

Dr. Sophie Cousin
Dr. Cousin is a medical oncologist within the Early Phase Trials department at Institut Bergonié. With over 230 publications and nearly 4,400 citations, her contributions span precision medicine, immunotherapy, and biomarker discovery. She’s co-authored studies on regorafenib plus avelumab (Regomune), as well as investigations in NSCLC immune exclusion and ctDNA-guided treatment strategies.
Together, Prof. Italiano and Dr. Cousin embody Institut Bergonié’s mission of translating scientific discovery into cutting-edge therapies — fostering early-phase trials, precision oncology, and multidisciplinary collaboration.

Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
