
KEYTRUDA-Chemotherapy Approved by NICE for Advanced Endometrial Cancer in England
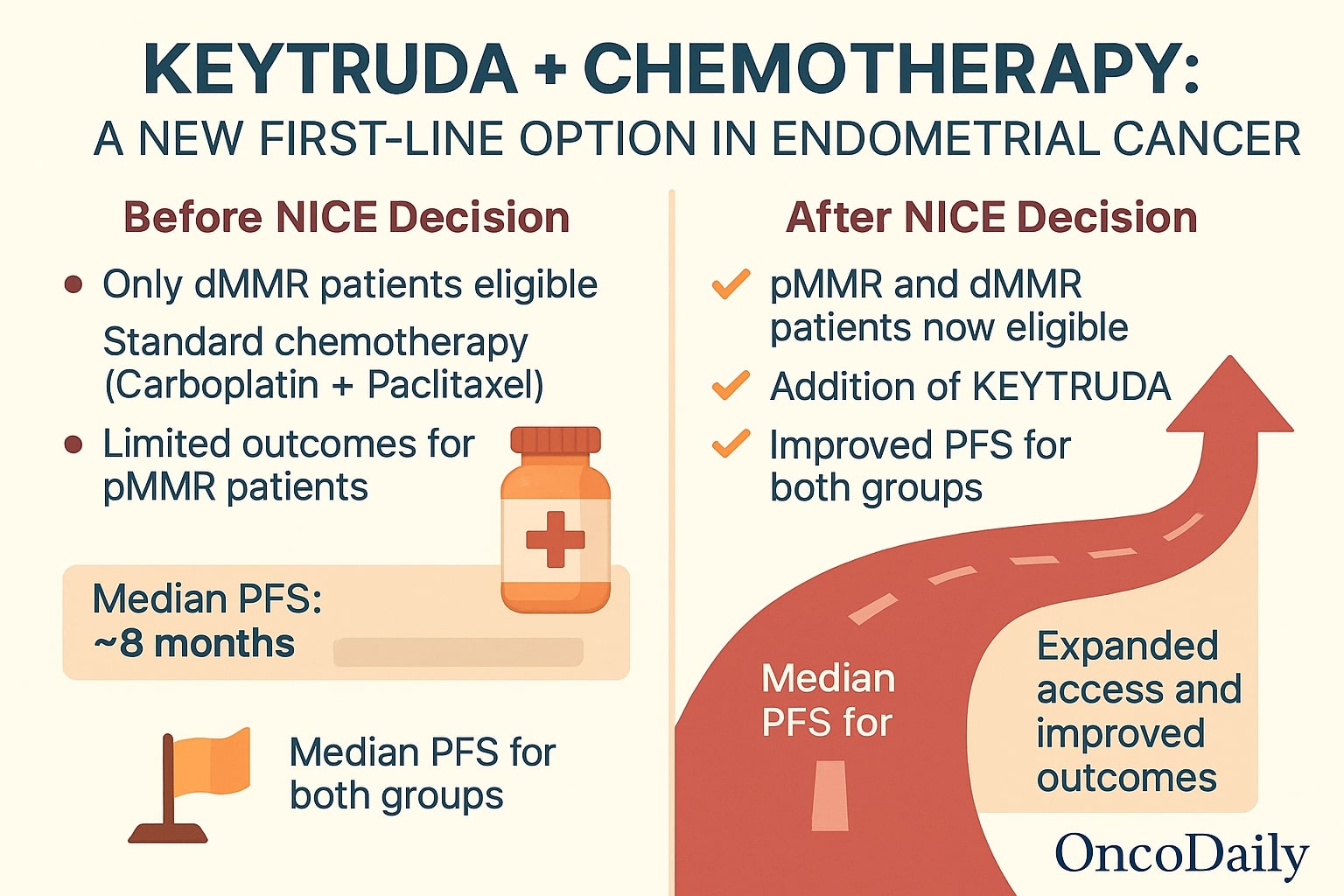
In a landmark decision for gynecologic oncology in the UK, the National Institute for Health and Care Excellence (NICE) has officially recommended the use of KEYTRUDA-chemotherapy as a first-line treatment for patients with advanced or recurrent endometrial cancer. This combination—pembrolizumab (KEYTRUDA) alongside carboplatin and paclitaxel—will now be available to adults with previously untreated primary advanced or recurrent disease, regardless of mismatch repair (MMR) status. The NICE decision marks a pivotal expansion of care, particularly for patients with mismatch repair proficient (pMMR) tumors, who previously had limited treatment options. Patients with both pMMR and dMMR tumors will now have access to this immunotherapy-based regimen, improving equity in therapeutic opportunity.
A First-Line Immunotherapy Option for All MMR Subtypes
Endometrial cancer is the most common gynecological cancer in the UK and ranks as the fourth most common cancer in women overall. With more than 9,700 new diagnoses annually in the UK alone, the need for more effective, durable treatment options has been an ongoing clinical priority.
Historically, first-line treatment options for endometrial cancer were limited to chemotherapy alone—most commonly carboplatin and paclitaxel. While this regimen provides temporary disease control, progression typically occurs within months, particularly in pMMR tumors, which are less responsive to immunotherapy than their dMMR counterparts.
Prior to the NICE decision, only patients with dMMR tumors—characterized by genomic instability and a high mutational burden—had access to immunotherapy in combination with chemotherapy. These patients make up only about 25–30% of the endometrial cancer population, leaving the majority (pMMR patients, ~70–75%) without access to this promising treatment modality.
The NICE recommendation now changes that paradigm.
Evidence Behind the Approval: KEYNOTE-868/GY018 Trial
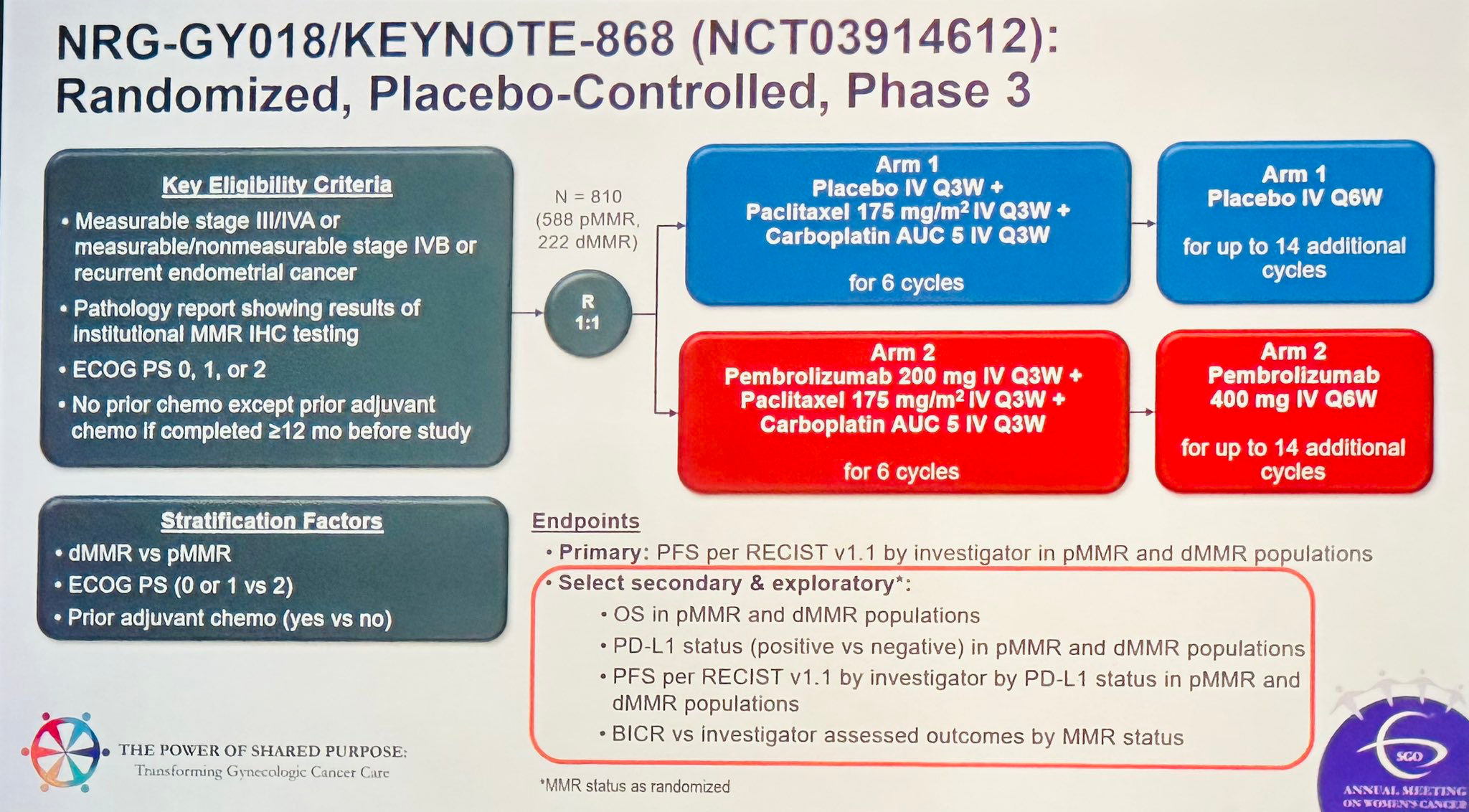
The recommendation is based on results from the KEYNOTE-868 / GY018 Phase III trial, which evaluated the efficacy of pembrolizumab in combination with carboplatin and paclitaxel versus chemotherapy alone.
The trial was designed with two co-primary cohorts:
- Cohort A: Patients with pMMR tumors
- Cohort B: Patients with dMMR tumors
In Cohort A (pMMR):
- Median progression-free survival (PFS) was 13.1 months with pembrolizumab + chemo vs 8.7 months with chemo alone
- Hazard Ratio (HR) for progression or death: 0.60 (95% CI: 0.45–0.80)
In Cohort B (dMMR):
- Median PFS was 13.1 months with the combination vs 7.6 months with chemo
- HR: 0.30 (95% CI: 0.19–0.48)
The combination therapy demonstrated statistically significant and clinically meaningful PFS benefits in both molecular subtypes, offering a critical new standard of care for a historically underserved group.
Clinical and Public Health Significance
The NICE recommendation marks a shift toward more equitable and personalized care for endometrial cancer patients. By removing the MMR status barrier to immunotherapy access, the decision:
- Provides a novel treatment pathway for the majority of patients with pMMR tumors.
- Enhances the durability of first-line treatment options beyond chemotherapy alone.
- Signals growing clinical confidence in combining immunotherapy with chemotherapy, even in less immunogenic tumor types.
It also underscores the increasing reliance on biomarker-driven treatment strategies and trial-backed real-world applicability.
Broader Implications and Next Steps
Endometrial cancer remains the most common gynecologic malignancy in the UK, with over 9,700 new diagnoses annually, according to Cancer Research UK. As treatment paradigms shift toward biomarker-informed strategies, the availability of KEYTRUDA-chemotherapy for both pMMR and dMMR patients reflects a broader trend toward inclusivity and personalization in oncology.
The approval aligns with growing global evidence supporting the integration of immune checkpoint inhibitors earlier in the treatment course of gynecologic cancers. Ongoing studies are investigating longer-term outcomes, overall survival, and potential combinations with other targeted therapies or maintenance strategies.
Read About Approval on NICE official website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
