
Paolo Tarantino: First Positive Phase 3 Trial for a Bispecific ADC
Paolo Tarantino, Clinical Research Fellow at Dana-Farber Cancer Institute, shared a post on X by Minhua Chu, Managing Partner at TransitionValue Partner, adding:
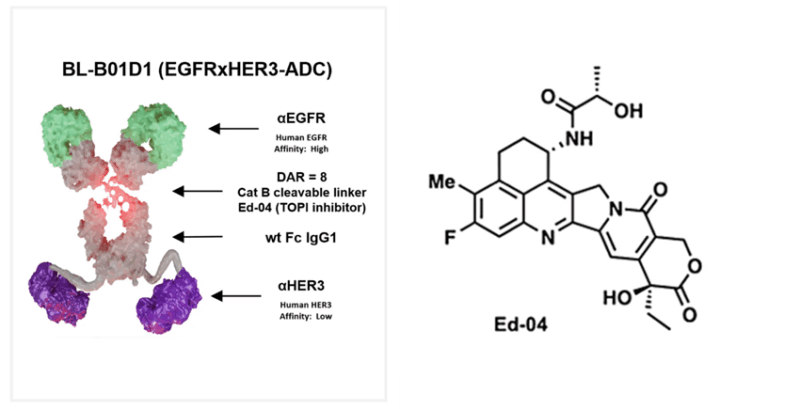
“First positive phase 3 trial for a bispecific ADC (EGFR/HER3 topo1 ADC), co-developed by Biokin Pharma and BMS. Bispecific ADCs are coming!”
Yüksel Ürün, Medical Oncology professor at Ankara University School of Medicine, also shared this post on X by Minhua Chu, adding:
“ADC race heats up!
Biokin’s EGFRxHER3 bispecific ADC, iza-bren, hits ≥1 primary endpoint in Ph3 NPC trial (vs chemo) — after 2+ lines and PD-1/L1. The next ADC wave is coming fast….”
Quoting Minhua Chu’s post:
“Biokin Pharma (SystImmune) announced that its EGFR/HER3 ADC, iza-bren (BL-B01D1), achieved the primary endpoint in the interim analysis of a Ph3 clinical trial (BL-B01D1-303) for the treatment of nasopharyngeal carcinoma.”
More posts featuring Paolo Tarantino.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
