
CONTACT-02 Trial 2025 Update: Atezolizumab Plus Cabozantinib in Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Prostate cancer that no longer responds to hormone therapy—known as metastatic castration-resistant prostate cancer (mCRPC)—is challenging to treat, especially after progression on novel hormonal agents (NHAs). The CONTACT-02 trial investigated whether combining immunotherapy with a targeted drug could improve outcomes for patients with this aggressive form of prostate cancer.
The trial tested atezolizumab (a PD-L1 inhibitor) combined with cabozantinib (a tyrosine kinase inhibitor) versus a second NHA (abiraterone or enzalutamide) in patients whose cancer had progressed after one prior NHA. The CONTACT-02 trial results were published in The Lancet Oncology in July 2025.
CONTACT-02 Trial Design
CONTACT-02 was a randomized, open-label, phase 3 study conducted across 184 sites in 24 countries, including Europe, North America, Asia-Pacific, and Latin America. Eligible men were 18 years or older with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. All participants had metastatic castration-resistant prostate cancer with measurable extrapelvic soft-tissue metastases that had progressed after one prior ARPI.
A total of 989 patients were screened between August 20, 2020, and June 7, 2023. Of these, 414 (42%) were ineligible, mainly due to the absence of measurable extrapelvic soft-tissue metastases (49% of screen failures). Ultimately, 575 patients were randomized 1:1 to receive cabozantinib (40 mg orally once daily) plus atezolizumab (1200 mg IV every three weeks) or an ARPI switch (abiraterone plus prednisone or enzalutamide). Randomization was stratified by the presence of liver metastases, prior docetaxel treatment, and disease status at the time of first ARPI initiation.
Patient Characteristics
Of the 575 randomized patients (289 in the combination arm and 286 in the ARPI switch arm), nearly half (48%) had visceral metastases, 23% had liver metastases, and 78% had bone metastases at baseline. Approximately 22% had received prior docetaxel in the metastatic hormone-sensitive prostate cancer (mHSPC) setting, and 74% had received their previous ARPI in the metastatic castration-resistant prostate cancer (mCRPC) setting, with a median duration of first ARPI treatment of 12 months.
Baseline demographics and disease characteristics were well balanced between groups, with median age around 71 years. Patients were predominantly White (approximately 75%), with a meaningful representation of Asian patients (~14%). The majority had Gleason scores ≥8 and ECOG scores of 0 or 1.
CONTACT-02 Trials’s Primary and Secondary Endpoints
The co-primary endpoints were:
- Radiographic progression-free survival (rPFS) per RECIST 1.1 and PCWG3 (blinded independent review committee).
- Overall survival (OS).
The primary efficacy analysis for progression-free survival was conducted in the first 400 randomized patients with a median follow-up of 11.8 months. The final overall survival analysis in the ITT population included all 575 patients with a median follow-up of 23.1 months.
Secondary endpoints included objective response rate, disease control rate, time to symptomatic skeletal events, time to chemotherapy use, prostate-specific antigen (PSA) response, and quality of life metrics.
CONTACT-02 Trial Results
The clinical impact of cabozantinib plus atezolizumab on progression-free survival, overall survival, and tumor response is discussed in detail below.
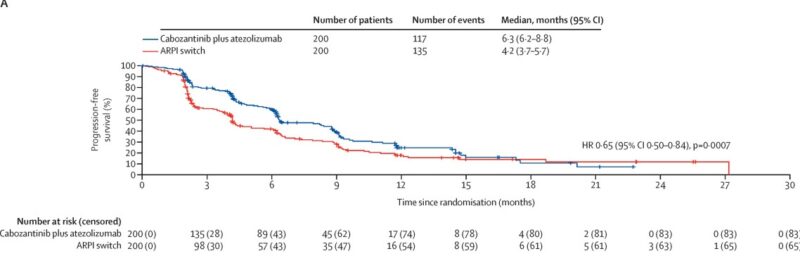
Progression-Free Survival
Cabozantinib plus atezolizumab significantly improved progression-free survival compared with the ARPI switch. Median PFS was 6.3 months (95% CI 6.2–8.8) in the combination group versus 4.2 months (95% CI 3.7–5.7) with ARPI switch, corresponding to a hazard ratio of 0.65 (95% CI 0.50–0.84, p=0.0007).
The PFS benefit was consistent across key subgroups including patients with liver metastases, prior docetaxel treatment, bone metastases, and regardless of whether the first ARPI was given in the mHSPC or mCRPC setting.
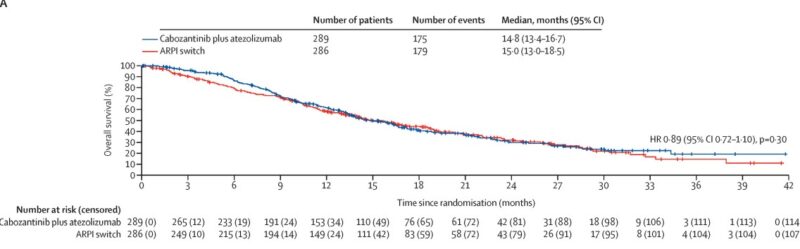
Overall Survival
At final analysis, overall survival was not significantly different between the two treatment arms. Median OS was 14.8 months (95% CI 13.4–16.7) for cabozantinib plus atezolizumab and 15.0 months (95% CI 13.0–18.5) for ARPI switch (HR 0.89, 95% CI 0.72–1.10, p=0.30). However, in an exploratory subgroup analysis, patients with liver metastases showed a trend toward improved survival with the combination (median OS 12.2 vs 7.1 months; HR 0.68, 95% CI 0.47–1.00).
Tumor Response
Among patients with measurable metastases at baseline, the objective response rate was higher in the cabozantinib plus atezolizumab group at 13% (33/257) compared to 6% (16/250) with ARPI switch. Disease control rates were 72% versus 53%, and fewer patients on combination therapy experienced progressive disease as their best response (17% vs 31%). Additionally, a greater proportion of patients receiving the combination had reductions in tumor size.
Additional Outcomes
Median time to symptomatic skeletal events was longer with cabozantinib plus atezolizumab (24.0 months) compared to ARPI switch (17.3 months), though not statistically significant. Time to chemotherapy initiation was significantly delayed in the combination arm (median 19.6 months vs 10.4 months, p<0.0001). PSA response rates and quality of life deterioration times were similar between groups. Safety In the safety population (284 patients per arm), median treatment duration was longer with cabozantinib plus atezolizumab (6.3 months) than ARPI switch (4.2 months).
Dose intensities remained high for all treatments. Adverse events of any grade were nearly universal (>99%) in the combination group and occurred in 92% of patients in the ARPI switch group. Grade 3–4 adverse events were more frequent with the combination (56%) compared to ARPI switch (26%). The most common severe adverse events in the combination group were hypertension (8%) and anemia (8%), while anemia (6%) was most common in the ARPI switch group.
Serious treatment-related adverse events occurred in 16% of patients receiving cabozantinib plus atezolizumab and 4% of those receiving ARPI switch. Diarrhea was the most frequent serious event in the combination arm (2%). Adverse events led to treatment discontinuation in 17% of patients on the combination and 15% on ARPI switch. No treatment-related deaths were reported. Immune-mediated adverse events of grade 3–4 severity occurred in 15% of patients on the combination therapy, including rare cases of encephalitis, hepatic failure, and rash. High-dose corticosteroids were required in 10% of these patients for management.
Atezolizumab Plus Cabozantinib
Cabozantinib is a small-molecule tyrosine kinase inhibitor (TKI) that targets a broad spectrum of receptor tyrosine kinases, including MET (hepatocyte growth factor receptor), VEGFR1-3 (vascular endothelial growth factor receptors), AXL, RET, KIT, and others. By inhibiting MET and VEGFR pathways, cabozantinib blocks tumor cell proliferation, angiogenesis, and metastasis. Additionally, its inhibition of AXL and other kinases helps remodel the tumor microenvironment, potentially making it more susceptible to immune attack. These broad mechanisms position cabozantinib as both an antiangiogenic and immunomodulatory agent.
Atezolizumab is a humanized monoclonal antibody that binds to PD-L1 (programmed death-ligand 1), a protein expressed on tumor cells and tumor-infiltrating immune cells. PD-L1 interacts with PD-1 receptors on T cells to inhibit their activity, allowing cancer cells to escape immune detection. By blocking PD-L1, atezolizumab reactivates T-cell–mediated immune responses against tumor cells, enhancing immune system activity and promoting anti-tumor effects.
This dual approach—targeting both the tumor’s growth signals and its ability to evade the immune system—makes the combination of cabozantinib and atezolizumab a promising therapeutic strategy in metastatic castration-resistant prostate cancer (mCRPC), particularly in patients with limited options after progression on androgen receptor pathway inhibitors.
Conclusion
The CONTACT-02 phase 3 trial demonstrated that cabozantinib plus atezolizumab significantly improves progression-free survival in men with metastatic castration-resistant prostate cancer and extrapelvic soft-tissue metastases after progression on ARPI therapy. Despite the lack of overall survival benefit in the overall population, the combination showed favorable tumor responses, delayed time to chemotherapy, and a tolerable safety profile. The survival trend seen in patients with liver metastases warrants further investigation.
This combination represents a novel non-androgen receptor targeted treatment option for a difficult-to-treat mCRPC population, with potential to fill an unmet need pending further confirmatory studies and longer follow-up.
The full article is available in The Lancet Oncology.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
