Comprehensive Chemotherapy Safety Guidelines: Protecting Patients, Staff, and Caregivers
Chemotherapy remains a cornerstone of cancer treatment despite the rise of targeted therapies and immunotherapy. Cytotoxic agents possess a narrow therapeutic index and systemic toxicity, necessitating strict precautions to minimize harm not only to patients but also to healthcare workers and caregivers who may be exposed through direct contact, aerosols, or contaminated waste. The potential for secondary exposure underscores the importance of comprehensive safety protocols. In modern oncology, ensuring safety is a multidimensional effort encompassing drug preparation, administration, environmental decontamination, waste disposal, and patient and caregiver education.
This article reviews current evidence-based chemotherapy precautions, highlighting clinical, occupational, and home-setting safety strategies. It examines best practices in drug handling, personal protective equipment (PPE), spill management, patient counseling, and the evolving regulatory and ethical landscape surrounding chemotherapy safety.
Rationale for Chemotherapy Precautions
Chemotherapeutic agents act primarily by targeting rapidly dividing cells, exerting their effects through mechanisms such as DNA intercalation, inhibition of DNA replication or repair, disruption of mitotic spindle formation, or induction of apoptosis. While these mechanisms are effective against malignant cells, their non-selectivity results in collateral damage to healthy proliferating tissues, including bone marrow, gastrointestinal mucosa, hair follicles, and renal tubular epithelium. This leads to a spectrum of adverse effects such as myelosuppression, mucositis, alopecia, nausea, and nephrotoxicity, all of which significantly impact patient quality of life and may necessitate dose reductions or treatment delays. Additionally, certain agents—particularly vesicants like anthracyclines or vinca alkaloids—pose a risk of extravasation, potentially causing severe local tissue injury if they leak into surrounding tissue during infusion.
Beyond patient toxicity, chemotherapy agents also pose substantial occupational hazards. Nurses, pharmacists, and other healthcare workers involved in drug preparation, administration, or waste handling are at risk of dermal absorption, inhalation of aerosolized particles, and accidental spills. Long-term low-dose exposure has been associated with reproductive toxicity, genotoxic effects, and dermatologic symptoms, particularly in inadequately protected or untrained personnel.
In recognition of these risks, major international organizations—including the Occupational Safety and Health Administration (OSHA), National Institute for Occupational Safety and Health (NIOSH), American Society of Clinical Oncology (ASCO), and European Society for Medical Oncology (ESMO)—have established comprehensive guidelines emphasizing the need for system-wide safety protocols, use of personal protective equipment (PPE), engineering controls, and institutional policies aimed at minimizing exposure and ensuring safe chemotherapy delivery. These guidelines underscore that the implementation of precautionary measures is not optional but essential for safeguarding both patients and healthcare providers.
Safe Handling and Preparation of Chemotherapy Agents
The compounding of chemotherapeutic agents requires meticulous control measures to protect healthcare personnel and prevent environmental contamination. Central to this process is the use of Class II Biological Safety Cabinets (BSCs), which provide both product and personnel protection through vertical laminar airflow and high-efficiency particulate air (HEPA) filtration. These cabinets are essential for maintaining a sterile field while minimizing aerosolized exposure to hazardous drugs during reconstitution or dilution.
In addition to BSCs, Closed System Drug-Transfer Devices (CSTDs) are increasingly recognized as a critical component of safe drug handling. CSTDs mechanically prevent the escape of hazardous drug vapors, droplets, and particles during preparation and administration. Their routine use, especially in high-volume oncology settings, has been associated with reduced environmental contamination and lower measurable drug exposure among pharmacy staff.
Personal Protective Equipment (PPE) further enhances safety. This includes chemotherapy-tested double gloves, impermeable gowns, eye protection, and respiratory protection when warranted. These measures are reinforced by institutional Standard Operating Procedures (SOPs) that outline the safe compounding of antineoplastic agents, including workflows, decontamination steps, and spill response protocols.
Trained oncology pharmacists are essential for ensuring compliance with these protocols. They possess specialized knowledge in drug pharmacology, compatibility, and safety, and are responsible for verifying dosages, handling materials, and overseeing the integrity of compounding processes.
Regulatory standards such as USP <800> (“Hazardous Drugs—Handling in Healthcare Settings”) provide a comprehensive framework for protecting healthcare workers. USP <800> mandates engineering controls, proper PPE, training, and procedures for receiving, storing, compounding, administering, and disposing of hazardous drugs. It also emphasizes environmental monitoring, including routine surface wipe sampling and air sampling, to assess contamination levels and ensure the effectiveness of implemented safety measures.
Administration Precautions in Chemotherapy Delivery
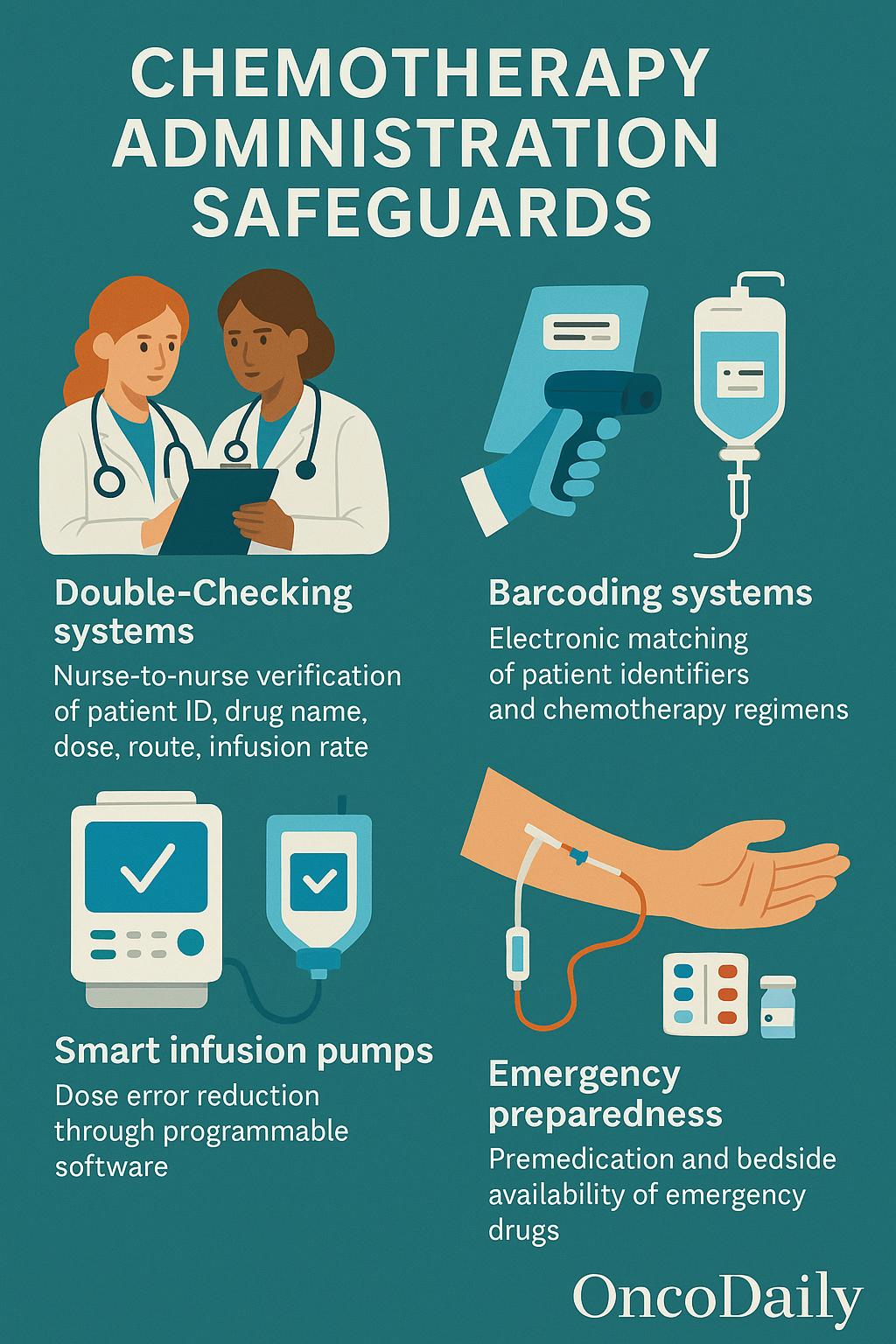
The administration of chemotherapeutic agents requires stringent safeguards to prevent dosing errors, ensure accurate delivery, and minimize risks to both patients and healthcare staff. One of the foundational practices is the implementation of protocolized double-checking systems, such as nurse-to-nurse verification, where two qualified staff members independently confirm the patient’s identity, drug name, dose, route, and infusion rate before administration. This process significantly reduces the likelihood of medication errors. The use of barcoding systems further enhances accuracy by electronically matching patient identifiers with prescribed chemotherapy regimens, reducing transcription and labeling mistakes.
Smart infusion pumps are routinely employed to deliver chemotherapeutic agents with precise control over flow rate and infusion duration. These devices often feature dose error reduction software (DERS), which cross-references the programmed parameters with institutional safety libraries to flag potential deviations.
Vascular access is another critical point of risk. For central venous catheters (CVCs), standard precautions include priming with non-drug fluids, secure line verification, and flushing with saline or heparin post-infusion to prevent clotting and drug residue. When peripheral IV access is used, it is essential to avoid vesicant agents—such as anthracyclines and vinca alkaloids—due to the high risk of extravasation, which can cause severe tissue necrosis. If peripheral administration is unavoidable, small veins, joints, or areas with poor tissue integrity should be avoided, and continuous monitoring of the site is essential.
In cases of extravasation, timely rescue protocols must be in place. For example, dexrazoxane is approved for anthracycline extravasation management, while cold or warm compresses, elevation, and surgical consultation may be required depending on the agent involved. Similarly, the potential for hypersensitivity reactions, especially with taxanes or platinum-based agents, necessitates premedication and the availability of emergency kits containing epinephrine, corticosteroids, and antihistamines at the bedside.
Personal Protective Equipment and Occupational Safety in Chemotherapy Handling
The use of Personal Protective Equipment (PPE) is essential in minimizing occupational exposure to hazardous chemotherapeutic agents. Standard PPE includes chemotherapy-tested gloves made from nitrile or neoprene, worn in double layers to provide a barrier against dermal absorption. These gloves should meet ASTM D6978 standards for chemotherapy drug resistance. In addition, disposable, lint-free, impermeable gowns with closed fronts, long sleeves, and tight-fitting cuffs are recommended to protect skin and clothing from contamination. Face shields or goggles are advised during procedures with a risk of splashing or aerosol generation, while respirators (e.g., N95 or PAPR devices)should be used in settings where aerosolized drug particles may be present, such as during cleaning of spills or drug compounding outside containment.
Proper use of PPE extends beyond simply donning the correct gear. Gloves must be changed every 30 minutes or immediately if torn or visibly contaminated, and after each administration or compounding session. Gowns should not be reused and must be discarded after each use, ideally in chemotherapy-designated waste containers. Hand hygienebefore and after glove use remains essential, even with full PPE.
Pregnant or breastfeeding healthcare workers are considered a high-risk group due to the potential teratogenic, mutagenic, and reproductive effects of antineoplastic drugs. Many institutional policies, supported by recommendations from NIOSHandASCO, advise the exclusion of these staff members from drug preparation, administration, or waste handling duties to prevent unintentional exposure. Institutions are encouraged to offer alternative work assignments and maintain confidential disclosure systems.
Recent research underscores the ongoing need for vigilance. A 2023 systematic review published in Journal of Occupational and Environmental Hygiene found that measurable levels of cyclophosphamide, ifosfamide, and fluorouracil were detectable on surfaces and in the urine of healthcare workers, even in settings with safety protocols. The presence of biomarkers such as DNA adducts and oxidative stress markers has been used to quantify low-level dermal and inhalation exposure. These findings highlight that PPE, while essential, must be part of a larger, integrated safety system that includes engineering controls, staff education, routine environmental monitoring, and adherence to established guidelines such as USP <800>, OSHA, and NIOSH recommendations.
Waste Disposal and Environmental Decontamination in Chemotherapy Safety
Proper disposal of hazardous drug waste is critical for minimizing occupational exposure and preventing environmental contamination. Chemotherapy-related waste is typically classified into two major categories: trace-contaminated wasteand bulk hazardous waste. Trace-contaminated waste includes materials such as used IV tubing, syringes, gloves, gowns, and empty vials or bags that previously held chemotherapy agents. These are considered low-volume residues but are still potentially hazardous and require specialized handling. Bulk hazardous waste, by contrast, includes unused or partially used chemotherapy drugs and items saturated with cytotoxic agents—this waste carries a significantly higher risk and is subject to stricter disposal protocols.
Both waste types must be discarded in clearly labeled, puncture-resistant chemotherapy waste containers, typically color-coded yellow or purple depending on local regulations. These containers should be leak-proof and compliant with hazardous materials handling standards, featuring appropriate cytotoxic warning labels. Absorbent pads are often placed at the bottom of these bins to contain accidental spills, and bins should be sealed when three-quarters full to prevent overflow or aerosol release.
Environmental safety also requires regular decontamination of compounding hoods, workstations, and administration areas. Detergent-based or sporicidal cleaning agents—specifically formulated to neutralize cytotoxic compounds—are recommended, followed by a disinfectant to ensure sterility. Decontamination should occur at least daily, after any spill, and in between the preparation of different agents. Spill kits must be readily available in all handling areas, containing PPE, absorbent materials, cleaning agents, and disposal bags, along with clearly written instructions for emergency response.
Regulatory oversight plays a key role in enforcing safe waste management. In the United States, the Environmental Protection Agency (EPA), under RCRA (Resource Conservation and Recovery Act), classifies many chemotherapy agents as U-listed hazardous wastes, requiring specific tracking and disposal procedures through certified hazardous waste handlers. Institutions must also comply with USP <800> guidelines and any additional protocols established by local health ministries or environmental safety agencies in their jurisdiction.
Together, these practices form a comprehensive system for mitigating environmental and occupational risks associated with chemotherapy waste, ensuring that the potential toxicity of these agents is controlled beyond their therapeutic use.
Patient and Caregiver Education in Chemotherapy Safety
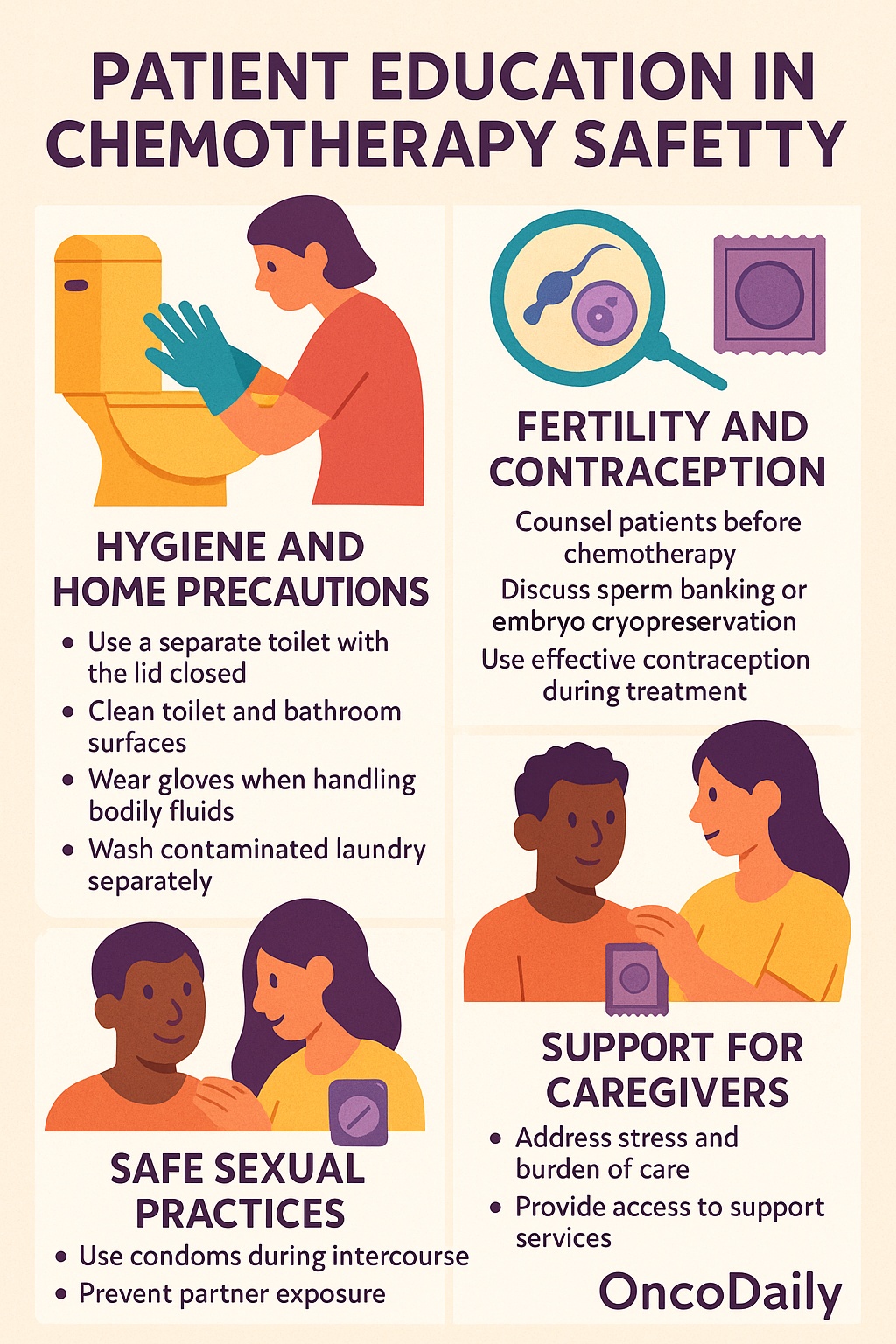
Effective patient education is a cornerstone of chemotherapy safety, particularly in the outpatient setting where patients return home with residual drug excretion posing potential risks to themselves and others. Many chemotherapeutic agents and their active metabolites are eliminated through urine, feces, vomit, and sweat, often for up to 48 to 72 hoursfollowing administration. Patients and caregivers must be explicitly informed about safe handling of these bodily fluids to minimize accidental exposure.
Hygiene and home safety precautions are essential during this period. When possible, patients should use a separate toilet, and the toilet lid should be closed before flushing to prevent aerosolization. In shared bathrooms, surfaces such as toilet seats and handles should be cleaned thoroughly after each use. Caregivers should wear disposable gloves when cleaning up bodily fluids, handling soiled linens, or taking care of patients with gastrointestinal side effects. Contaminated clothing or bed linens should be laundered separately using hot water and detergent, and direct contact with skin should be avoided.
Beyond immediate exposure risks, patients must also receive education on fertility preservation and contraception. Many cytotoxic agents are gonadotoxic, posing long-term risks to reproductive health. Patients of reproductive age should be counseled about fertility preservation options (e.g., sperm banking, oocyte or embryo cryopreservation) prior to starting treatment. Both male and female patients should be advised to use effective contraception during treatment and for several months afterward, as chemotherapy can harm developing embryos. Additionally, safe sexual practicesare encouraged, including the use of condoms during intercourse to prevent partner exposure through semen or vaginal secretions.
Equally important is the protection and support of caregivers, who often play a vital role in the management of patients undergoing chemotherapy. Educational materials should include not only physical safety measures but also guidance on emotional and psychological support, recognizing that the outpatient nature of many regimens shifts a significant caregiving burden to families. Addressing caregiver stress, providing access to support services, and involving them in treatment education can enhance both patient safety and caregiver well-being.
Chemotherapy Precautions in Specific Care Settings
- Outpatient Infusion Units: Outpatient infusion units are characterized by high patient volumes and rapid turnover, which can increase the risk of accidental exposures and procedural errors. Tight scheduling and overlapping infusions demand streamlined workflows and effective communication among staff. Workflow optimization, such as standardized protocols, electronic checklists, and pre-visit medication verification, plays a crucial role in maintaining safety. The presence of oncology nurse navigators is especially valuable—they coordinate care, educate patients, and serve as liaisons between clinical teams, thereby reducing confusion and enhancing both efficiency and safety.
- Inpatient Wards: In hospital settings, where chemotherapy may be administered to acutely ill or neutropenic patients, precautions must extend beyond administration to include the safe handling of body fluids, excreta, and contaminated linens. Strict adherence to PPE protocols is essential not only for nursing staff but also for cleaning and environmental services personnel, who may encounter hazardous waste during room cleaning or linen changes. Effective coordination between clinical and support teams ensures that all staff are informed, trained, and equipped to manage chemotherapy-related hazards safely and consistently across shifts.
- Home-Based Chemotherapy or Oral Agents: With the increasing use of oral chemotherapy agents such as capecitabine and targeted therapies, more patients are receiving treatment at home, shifting responsibility for safety to patients and caregivers. Proper storage in secure, labeled containers, timely and accurate dosing, and disposal of unused medications are critical to prevent unintentional exposure or misuse. Institutions are adopting telemonitoring tools and remote toxicity reporting protocols to ensure ongoing safety and early detection of adverse effects. Patient education materials and virtual support from clinical teams help maintain treatment adherence while minimizing risks in the home setting.
Chemotherapy Precautions During Pandemics and Infectious Outbreaks
The COVID-19 pandemic exposed critical vulnerabilities in the delivery of chemotherapy, particularly related to PPE shortages, heightened aerosol-related risks, and the profound immunosuppression experienced by oncology patients. Cytotoxic chemotherapy compromises both innate and adaptive immunity, placing cancer patients at significantly increased risk of severe outcomes from viral infections. At the same time, global supply chain disruptions led to insufficient availability of protective equipment, posing additional hazards for healthcare workers administering chemotherapy in both outpatient and inpatient settings.
To address these challenges, oncology centers rapidly implemented adaptive strategies aimed at reducing exposure risks while maintaining continuity of care. These included the expansion of home-based infusion services for select regimens, minimizing patient traffic in healthcare facilities. Pre-visit symptom screening, staggered appointment times, and restructured waiting areas helped reduce the potential for cross-contamination in high-volume clinics. The pandemic also accelerated the adoption of teleoncology, allowing providers to conduct follow-up visits, toxicity assessments, and treatment planning remotely, preserving critical care continuity with reduced in-person contact.
Emerging evidence from international studies during the pandemic suggests that these adaptive models were largely effective. A 2021 multicenter cohort study published in JAMA Oncology demonstrated no significant increase in cancer progression or mortality among patients who received modified chemotherapy schedules or remote monitoring, while staff infection rates declined with appropriate use of screening and PPE prioritization. These findings underscore the feasibility of integrating telehealth, decentralized care, and flexible protocols into long-term chemotherapy delivery models—enhancing resilience not only during public health emergencies but also in routine oncology practice.
Legal, Regulatory, and Ethical Considerations in Chemotherapy Safety
Chemotherapy safety is not only a clinical obligation but also a legal and institutional responsibility. Patients must provide informed consent acknowledging potential treatment risks, while healthcare institutions are responsible for maintaining and documenting adherence to safety protocols related to hazardous drug handling and staff protection. This includes training records, spill response procedures, and environmental monitoring.
Laws in many countries, including OSHA standards in the U.S., mandate reporting of occupational exposures and protect whistleblowers who raise concerns about unsafe practices. Despite these frameworks, significant ethical challenges persist, particularly regarding equitable access to PPE and safety infrastructure. In many low- and middle-income countries, staff may lack even basic protective equipment, underscoring global disparities in chemotherapy safety. Ethical oncology practice requires ongoing advocacy to ensure that all healthcare workers—regardless of geography—have access to the resources and protections necessary to safely deliver cancer care.
Future Directions in Chemotherapy Safety
Innovations in oncology practice are driving a new era of chemotherapy safety. Robotic compounding systems are increasingly being used in pharmacy settings to reduce human error and minimize staff exposure to hazardous drugs. These automated systems ensure precise dosing and sterile preparation while enhancing reproducibility. Similarly, AI-based error detection tools—such as real-time prescription verification algorithms and smart infusion programming—are being integrated to identify inconsistencies or risks before administration occurs. Wearable sensors capable of monitoring infusion parameters and detecting early signs of extravasation offer an added layer of patient safety during drug delivery. In parallel, next-generation PPE technologies are being developed with improved barrier protection, comfort, and durability, especially under high-use conditions.
Globally, there is a growing consensus on the need for harmonized safety standards, particularly as chemotherapy use expands in low- and middle-income countries (LMICs). Several international oncology organizations have called for inclusion of LMICs in implementation studies to adapt evidence-based safety practices to resource-constrained settings without compromising protection.
Despite these advancements, research gaps remain. The long-term effects of chronic low-dose occupational exposureto cytotoxic drugs are still not fully understood, particularly with regard to genotoxicity and reproductive outcomes. Additionally, there is limited guidance on the optimal safety protocols for caregivers in home settings, and few real-world safety audits systematically evaluate the effectiveness of implemented precautions across institutions. Addressing these gaps will be essential to building a comprehensive, equitable, and evidence-driven framework for chemotherapy safety in the years ahead.
You Can Watch More on OncoDaily Youtube TV
FAQ
What is chemotherapy safety?
It refers to protocols that minimize risks for patients and healthcare workers during chemotherapy use.
Why is PPE important in chemotherapy?
PPE protects staff from hazardous drug exposure via skin or inhalation.
What are closed system drug-transfer devices (CSTDs)?
CSTDs prevent escape of chemotherapy vapors and reduce contamination.
How is chemotherapy waste managed?
Waste is sorted into trace and bulk categories and disposed of in certified containers.
Are oral chemotherapies hazardous?
Yes, they still require safe handling due to systemic toxicity.
What is USP ?
A U.S. standard for safe handling of hazardous drugs in healthcare settings.
How can caregivers stay safe at home?
Use gloves, wash soiled linens separately, and clean shared toilets after each use.
Is chemotherapy safety different during pandemics?
Yes, precautions include telehealth, PPE prioritization, and home infusion models.
What happens if a chemo drug leaks under the skin?
It’s called extravasation; immediate action is required to prevent tissue damage.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023