
World’s First Successful Personalized CRISPR Therapy Administered by CHOP and Penn Medicine
In a landmark moment for medicine and genetic science, a team from the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania’s Perelman School of Medicine has successfully treated a child with a rare genetic disorder using a personalized CRISPR-based gene editing therapy—the first such case in the world.
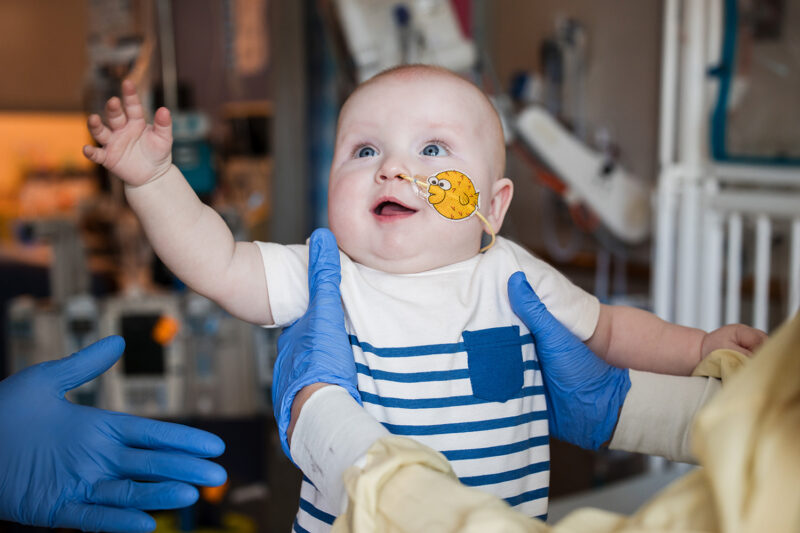
The groundbreaking therapy was developed for KJ, an infant diagnosed with severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, a rare and life-threatening metabolic condition. The condition disrupts the urea cycle, preventing the body from removing toxic levels of ammonia, posing a constant risk of brain damage or death.

From Diagnosis To Treatment In Record Time
Led by Dr. Rebecca Ahrens-Nicklas, Director of CHOP’s Gene Therapy for Inherited Metabolic Disorders (GTIMD) Frontier Program, and Dr. Kiran Musunuru, the Barry J. Gertz Professor at Penn Medicine, the team developed a bespoke base editing therapy targeting KJ’s specific CPS1 gene variant. Within six months of identifying the variant, the therapy was manufactured and administered using lipid nanoparticles to deliver the edited gene directly to KJ’s liver.
Treatment Timeline & Results
- KJ received his first infusion in February at around 6–7 months of age.
- Two additional follow-up doses were administered in March and April.
- No serious side effects reported.
- Significant improvements noted: better protein tolerance, reduced medication needs, and ability to recover from common illnesses without ammonia buildup.
Published in The New England Journal of Medicine and presented at the ASGCT 2024 Annual Meeting, this case exemplifies the potential of precision gene editing for ultra-rare diseases that previously had no viable treatment options.

Why It Matters:
While CRISPR-based therapies have been approved for more common diseases like sickle cell anemia and beta-thalassemia, this case demonstrates the scalability of personalized gene editing for individual mutations—a transformative leap forward for millions of patients living with rare genetic diseases.
“While KJ is just one patient, we hope he is the first of many to benefit from a methodology that can be scaled to fit an individual patient’s needs,” – said Dr. Ahrens-Nicklas.
“The promise of gene therapy that we’ve heard about for decades is coming to fruition. “It’s going to utterly transform the way we approach medicine.” – added Dr. Kiran Musunuru.
A Family’s Hope:
KJ’s parents, Nicole and Kyle Muldoon, courageously embraced the experimental therapy, not only for their son but also for the many families who might benefit from this innovative approach in the future. Now home, reunited with his siblings, KJ represents a symbol of hope for the future of rare disease treatment.
Supported by: NIH Somatic Cell Genome Editing Program, CHOP Research Institute, and in-kind contributions from industry collaborators including Acuitas Therapeutics and Integrated DNA Technologies.
This case sets the stage for a new era of truly personalized medicine—an inspiring reminder of how research, collaboration, and compassion can change the future for patients once left with no options.
For more stories like this and the latest breakthroughs in cancer and genetic research, follow OncoDaily across all platforms.
Written by Md Foorquan Hashmi, MD, Sr. Editor, OncoDaily: India Bureau
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
