
Amol Akhade: Aumolertinib and Chemotherapy, A New Challenger to Osimertinib?
Amol Akhade, Senior consultant medical oncologist and hemato-oncologist at Suyog Cancer Clinics and Reliance Hospitals, shared a post on LinkedIn:
“Aumolertinib and Chemotherapy: A New Challenger to Osimertinib? (Insights from AACR25)
The landscape of first-line treatment for EGFR-mutant NSCLC continues to evolve!
At AACR2025, the AENEAS2 trial evaluating Aumolertinib and Platinum–Pemetrexed showcased remarkable results:
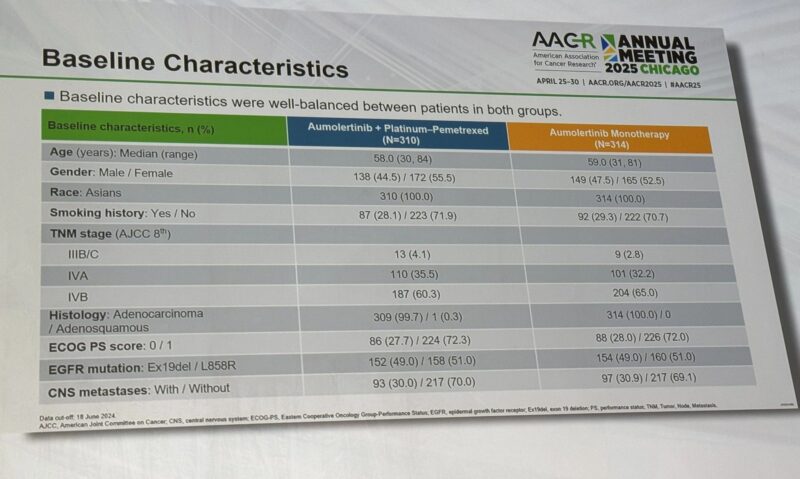
Median PFS:
- Aumolertinib and Chemo: 28.9 months.
- Aumolertinib Monotherapy: 18.9 months.
- (HR 0.47, 95% CI 0.37–0.60).
Early OS trend:
- Hazard Ratio for Overall Survival: 0.44.
- (Median OS not yet reached).
Strong Subgroup Benefits:
Consistent efficacy across gender, smoking history, ECOG PS, CNS metastases, and EGFR mutation types (Ex19del and L858R). Especially powerful in younger patients, smokers, and Exon19 deletion patients.
CNS Metastasis Control:
Maintained with a Hazard Ratio of 0.56 for patients with baseline brain mets.
Comparison with FLAURA2 (Osimertinib and Chemo):
Median PFS:
- Aumolertinib and Chemo = 28.9 months (HR 0.47).
- Osimertinib and Chemo = 25.5 months (HR 0.62).
Overall Survival Trend:
- Aumolertinib and Chemo = HR 0.44 (early strong signal).
- Osimertinib and Chemo = HR ~0.79 (not statistically significant yet).
CNS Metastases Control:
- Aumolertinib = HR 0.56
- Osimertinib = HR ~0.58
Population:
- Aumolertinib trial = 100% Asian.
- Osimertinib trial = Global (~62% Asian).
Key Takeaways:
Aumolertinib and Chemo is setting a new bar for PFS in EGFR-mutant NSCLC.
Early OS signals appear stronger than in FLAURA2. Subgroup consistency and CNS activity are both encouraging.
Pending: Full quality of life and global applicability data.”
More posts featuring Amol Akhade.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
