What Happens After Immunotherapy Ends?
Immunotherapy has emerged as a transformative approach in cancer treatment, harnessing the body’s immune system to recognize and destroy malignant cells. Unlike traditional therapies, immunotherapy offers the potential for durable responses and improved survival in various cancers, including melanoma, lung, kidney, and certain gastrointestinal tumors. However, with its growing use comes the need to understand the post-treatment experience of patients. This article focuses on life after immunotherapy, exploring common side effects, the recovery process, and the long-term outlook for survivors.
Recent studies have shown that up to 30–50% of patients experience immune-related adverse events, some of which may persist beyond treatment completion (Postow et al., 2018; ASCO, 2023). As survivorship increases, understanding these outcomes becomes essential for clinicians and patients alike. This article aims to provide an evidence-based overview of the physical, emotional, and clinical dimensions of recovery following immunotherapy.
How Does Immunotherapy Work?
Immunotherapy works by enhancing the body’s immune system to recognize and attack cancer cells. Unlike traditional therapies that directly target tumor cells, immunotherapy activates or modifies immune components—particularly T lymphocytes—to identify malignant cells and eliminate them more effectively and selectively.
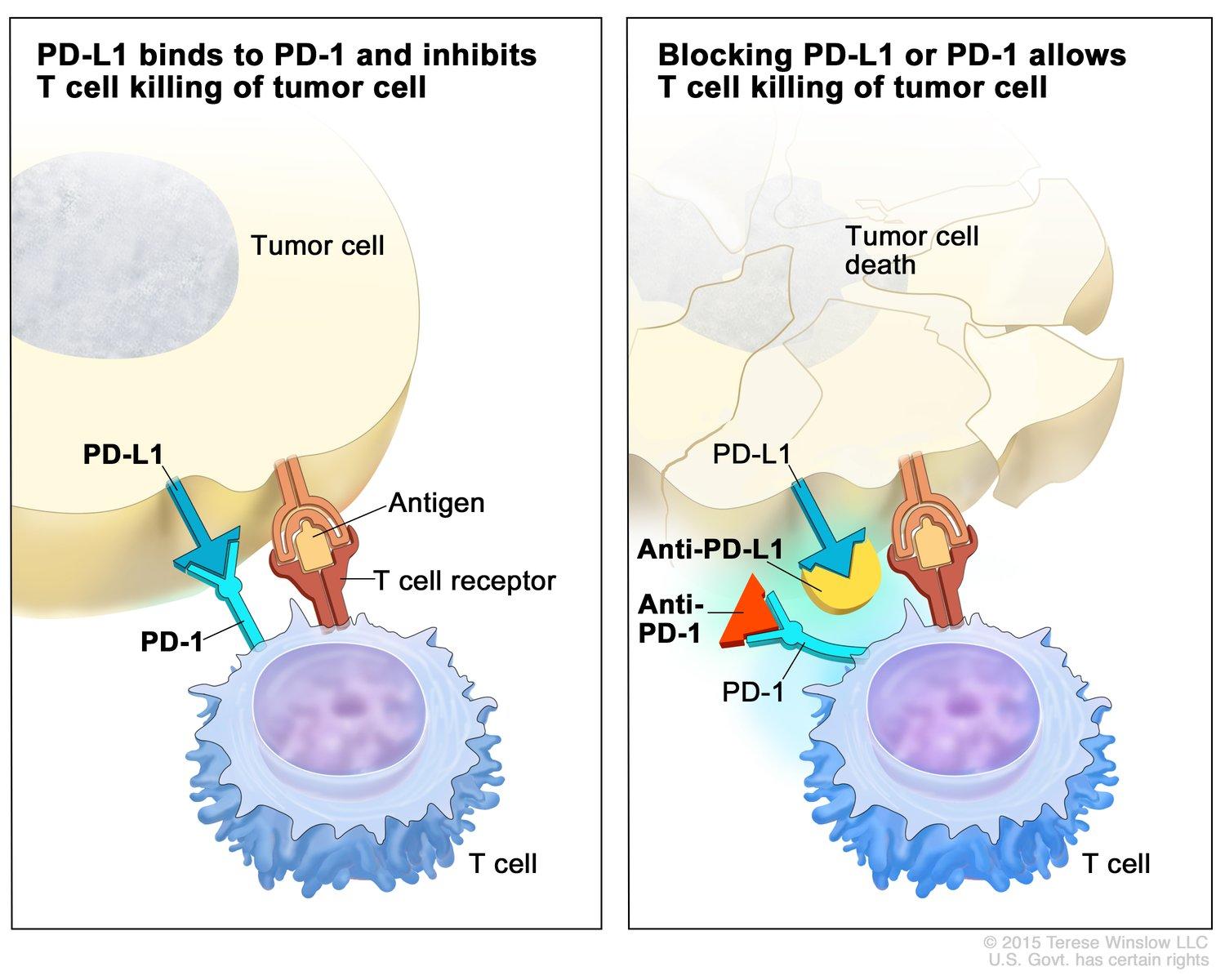
Cancer cells often evade immune destruction by exploiting immune checkpoints—regulatory pathways that normally prevent autoimmunity but are hijacked by tumors to suppress immune responses. Immunotherapy drugs called immune checkpoint inhibitors block these pathways, releasing the immune system’s inhibitory brakes and restoring T-cell activity.
Among the most established checkpoint inhibitors are:
-
PD-1 inhibitors like pembrolizumab (Keytruda) and nivolumab (Opdivo), which prevent the interaction between PD-1 on T cells and PD-L1 on tumor cells.
-
PD-L1 inhibitors such as atezolizumab (Tecentriq) and durvalumab (Imfinzi), which block PD-L1 directly.
-
CTLA-4 inhibitors like ipilimumab (Yervoy), which enhance early-stage T-cell activation in lymphoid tissues.
More recently, next-generation immunotherapies are being developed to expand the effectiveness of immune-based treatments, especially in tumors that are less responsive to conventional checkpoint blockade. One such example is the combination of balstilimab and botensilimab.
-
Balstilimab is a novel anti–PD-1 monoclonal antibody that works similarly to pembrolizumab and nivolumab by enhancing T-cell responses against tumors. It has demonstrated activity in several solid tumors, including cervical, anal, and head and neck cancers.
-
Botensilimab is an innovative next-generation anti–CTLA-4 antibody engineered with enhanced Fc region modifications to increase immune activation while minimizing toxicity. Unlike traditional CTLA-4 inhibitors, botensilimab selectively modulates effector and regulatory T-cell populations and is designed to improve safety and efficacy.
The combination of balstilimab and botensilimab has shown promising early clinical results, particularly in immunologically “cold” tumors such as microsatellite stable (MSS) colorectal cancer and non–small cell lung cancer that are typically unresponsive to single-agent immunotherapy. Early-phase trials suggest this combination can induce T-cell infiltration into tumors and overcome local immunosuppression, leading to durable antitumor responses.
Side Effects Management
While immunotherapy has transformed the treatment landscape for many cancers, it also introduces a unique spectrum of side effects, often termed immune-related adverse events (irAEs). These side effects arise as a result of the immune system becoming overactive and mistakenly targeting healthy tissues, and they can affect virtually any organ system. Though many irAEs are manageable and reversible, early recognition and intervention are essential to prevent long-term complications and to preserve quality of life.
One of the most frequently reported side effects is fatigue, which can range from mild tiredness to profound exhaustion that interferes with daily activities. Fatigue may result from chronic inflammation, hormonal imbalances (such as hypothyroidism), or the cumulative burden of cancer and its treatment. Management strategies include energy conservation techniques, regular light exercise such as walking or stretching, adequate hydration, and sleep hygiene. In some cases, evaluation for underlying endocrine dysfunction or anemia is warranted, as correcting these issues can significantly improve symptoms. Skin reactions are also common, presenting as rash, itching, dryness, or vitiligo. These typically appear within the first few weeks of treatment but can persist or recur. While most dermatologic reactions are mild, some may progress to more serious conditions such as Stevens-Johnson syndrome or toxic epidermal necrolysis, though these are rare. For mild to moderate rashes, topical corticosteroids and antihistamines are effective first-line treatments. Severe or widespread reactions may require systemic steroids and temporary discontinuation of immunotherapy. Regular skin moisturizing and sun protection are essential for prevention and comfort.
Gastrointestinal side effects, such as diarrhea, abdominal pain, or colitis, are particularly associated with checkpoint inhibitors targeting CTLA-4. Diarrhea may be mild and self-limited, but persistent or severe symptoms may indicate immune-mediated colitis, which can lead to dehydration, electrolyte imbalances, or even bowel perforation in extreme cases. Management includes ruling out infectious causes, initiating anti-inflammatory medications like corticosteroids, and in refractory cases, considering infliximab or vedolizumab for immunosuppression. Patients are advised to maintain a bland diet during flares and to report symptoms early for prompt management. Other common issues include endocrinopathies (such as thyroiditis, hypophysitis, or adrenal insufficiency), joint pain, and pulmonary toxicity like pneumonitis. These require specialized monitoring and, in some cases, hormone replacement therapy or immunosuppressive treatment. Regular blood work, hormonal panels, and imaging may be used to detect and monitor these effects.
Importantly, improving quality of life during and after immunotherapy involves not only medical management of symptoms but also psychological and social support. Counseling, patient education, nutrition services, and peer support groups play vital roles in helping patients cope with both the physical and emotional challenges of immunotherapy. As survivorship grows, integrating supportive care into routine oncology practice is essential to ensure that the benefits of treatment are matched by a good quality of life during and beyond recovery.

Does Thyroid Recover After Immunotherapy?
Thyroid dysfunction is among the most frequently reported endocrine side effects of immune checkpoint inhibitors, particularly those targeting programmed cell death protein 1 (PD-1) and its ligand PD-L1, such as pembrolizumab and nivolumab. These agents can trigger immune-related thyroiditis, resulting from immune activation that leads to inflammation and destruction of thyroid tissue. This condition often manifests in a biphasic pattern—an initial phase of thyrotoxicosis, due to the release of preformed thyroid hormones, followed by a transition to hypothyroidism as the thyroid gland is depleted or permanently damaged. According to the American Society of Clinical Oncology (ASCO) and NCCN Guidelines, the incidence of thyroid dysfunction ranges from 5% to 20% in patients treated with PD-1/PD-L1 inhibitors, with even higher rates in those receiving combination therapy with CTLA-4 inhibitors such as ipilimumab. While the thyrotoxic phase is typically transient and may resolve spontaneously or with supportive care (e.g., beta-blockers for symptomatic relief), the ensuing hypothyroidism is often permanent. Recovery of normal thyroid function is unlikely once the hypothyroid phase has been established, as significant glandular damage may have occurred.
Management of post-immunotherapy hypothyroidism generally involves lifelong thyroid hormone replacement therapy, most commonly with levothyroxine. Routine monitoring of thyroid-stimulating hormone (TSH) and free thyroxine (T4) levels is recommended prior to treatment initiation and periodically during therapy, even in asymptomatic patients, to allow early detection and intervention. Patients with pre-existing autoimmune thyroid disease are at slightly increased risk for immune-related thyroid dysfunction, but the condition can develop de novo in individuals with no history of thyroid abnormalities. Importantly, the development of thyroid dysfunction does not necessitate discontinuation of immunotherapy, provided that the endocrine disturbance is properly managed. In fact, some retrospective studies have suggested a possible association between immune-related thyroiditis and improved tumor response, although this remains an area of ongoing research.
Patient education is critical, as symptoms such as fatigue, weight gain or loss, cold or heat intolerance, and mood changes may overlap with those of cancer or other treatment-related effects. Referral to endocrinology may be appropriate in cases of complex or severe endocrine complications.
Long-term Monitoring and Follow-up Care
Regular follow-up and monitoring are critical components of post-immunotherapy care. As immunotherapy can lead to durable responses—even long after treatment has ended—it is essential to continuously assess both disease status and treatment-related effects. Ongoing surveillance allows clinicians to detect cancer recurrence early, manage late-onset immune-related adverse events, and monitor overall health and quality of life.
The need for structured follow-up is underscored by the fact that immune checkpoint inhibitors may produce delayed responses or pseudo-progression, where tumors appear to grow before regressing. Additionally, immune-related toxicities can emerge weeks to months after therapy, even in patients no longer actively receiving treatment. For this reason, follow-up care must be both oncologic and immunologic in focus. A typical post-immunotherapy monitoring plan includes:
-
Imaging studies: Routine imaging—such as CT scans, MRI, or PET scans—is used to evaluate tumor status and detect early signs of recurrence. The frequency of imaging depends on the cancer type, initial disease stage, and risk of relapse, but scans are commonly performed every 3 to 6 months during the first few years post-treatment.
-
Laboratory tests and blood work: Regular blood tests assess organ function (e.g., liver enzymes, kidney function), endocrine markers (such as TSH, cortisol, and glucose), and inflammatory parameters. These help identify immune-related adverse events that may be subclinical, including thyroiditis, hepatitis, or adrenal insufficiency.
-
Physical exams and symptom review: At each visit, clinicians evaluate for signs and symptoms of recurrence or immune-related toxicities. Fatigue, rash, diarrhea, or joint pain may indicate late effects requiring further work-up.
-
Quality of life assessments: Psychological and functional assessments are often integrated into survivorship care to address mental health, fatigue, and the social impact of cancer and its treatment.
In addition to clinical surveillance, patients are encouraged to report new or persistent symptoms promptly, as early recognition of immune-related side effects significantly improves outcomes. In cases of suspected recurrence, biopsies or molecular testing may be required to differentiate true progression from immune-mediated inflammation.
Life Expectancy and Long-term Outcomes
mmunotherapy has markedly improved survival outcomes in a range of advanced cancers, offering sustained remission or long-term control in cases once deemed incurable. Landmark studies have now demonstrated that immune checkpoint inhibitors can lead to durable responses and significantly extended life expectancy in selected patient populations.
In advanced melanoma, the pivotal CheckMate 067 trial (Larkin et al., New England Journal of Medicine, 2015; updated 10-year follow-up in 2023) investigated the combination of nivolumab (anti–PD-1) and ipilimumab (anti–CTLA-4). Among 945 patients, the median overall survival for those receiving combination therapy was 72.1 months (6 years), with 49% of patients alive at 6.5 years. This is a striking contrast to historical 5-year survival rates of around 15–20% with conventional therapies. These results confirm that dual checkpoint inhibition can provide long-term survival for nearly half of advanced melanoma patients. In non-small cell lung cancer (NSCLC), the KEYNOTE-010 trial (Herbst et al., Lancet, 2016; long-term update 2023) evaluated pembrolizumab versus docetaxel in patients with previously treated, PD-L1-positive NSCLC. Among patients who completed the full 2 years of pembrolizumab treatment (35 cycles), the 3-year overall survival rate was reported as 83%, and importantly, 52.4% of those retreated after disease progression achieved a renewed objective response (Garon et al., J Immunother Cancer, 2023).
Similarly, in triple-negative breast cancer (TNBC), the KEYNOTE-522 trial (Schmid et al., New England Journal of Medicine, 2020; 5-year update 2024) demonstrated that the addition of pembrolizumab to neoadjuvant chemotherapy, followed by adjuvant pembrolizumab, significantly improved long-term outcomes in early-stage TNBC. The 5-year overall survival was 86.6% in the pembrolizumab arm, compared to 81.7% in the placebo arm, underscoring the potential of immunotherapy in earlier-stage, aggressive cancers. One of the most compelling demonstrations of long-term benefit comes from the 10-year follow-up of CheckMate 067, where over half of patients with advanced melanoma treated with nivolumab + ipilimumab remained alive a decade after treatment initiation (Larkin et al., ESMO 2023; The Guardian, 2024). Such survival durations were once unthinkable in the context of metastatic disease.
These outcomes highlight the durability of immunotherapy, which can not only extend life but also, in some cases, functionally “cure” subsets of patients. Long-term survivorship is now a reality for many, shifting the focus of care to managing late effects, quality of life, and post-treatment monitoring.
Factors Influencing Long-term Outcomes
While immunotherapy has revolutionized cancer treatment and improved survival in numerous malignancies, its effectiveness and long-term outcomes are influenced by a variety of clinical, biological, and patient-related factors. Understanding these determinants is essential to optimize patient selection, personalize treatment strategies, and provide accurate prognostic information.
One of the most critical factors is the type of cancer being treated. Immunotherapy has shown remarkable efficacy in tumors with high immunogenicity, such as melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, Hodgkin lymphoma, and certain gastrointestinal tumors like microsatellite instability-high (MSI-H) colorectal cancer. These cancers tend to express higher levels of neoantigens or PD-L1, making them more susceptible to immune checkpoint blockade. In contrast, tumors that are “immune-cold” or have low tumor mutational burden (TMB)—such as pancreatic or prostate cancer—often demonstrate limited responsiveness, although research is ongoing to improve outcomes in these settings. The stage at diagnosis also plays a pivotal role. Patients diagnosed at an early stage, particularly those receiving immunotherapy as adjuvant or neoadjuvant treatment, tend to have better long-term outcomes compared to those with metastatic or unresectable disease. For example, in early-stage non-small cell lung cancer, adjuvant immunotherapy has shown promising disease-free survival benefits, while patients with widespread metastatic burden may experience shorter remissions and require ongoing therapy.
Additionally, the molecular profile of the tumor is a key determinant. Tumors harboring PD-L1 expression, high TMB, MSI-H status, or deficient mismatch repair (dMMR) are more likely to respond favorably to checkpoint inhibitors. Conversely, patients with driver mutations such as EGFR or ALK rearrangements in lung cancer are less responsive to immunotherapy and may benefit more from targeted therapies. Overall patient health and performance status are also major contributors to long-term outcomes. Patients with good Eastern Cooperative Oncology Group (ECOG) performance status (0–1) tend to tolerate immunotherapy better and derive greater benefit. In contrast, individuals with significant comorbidities, chronic immunosuppression, or organ dysfunction may experience reduced efficacy or higher toxicity. Additionally, age alone is not a contraindication, but elderly patients may require closer monitoring for immune-related adverse events.
The development of immune-related side effects has also been associated with improved treatment response in some studies. This suggests that a more robust immune activation may correlate with better tumor control, although severe toxicity may require treatment discontinuation and can negatively impact quality of life. Finally, access to care, timely diagnosis, and adherence to follow-up all influence long-term outcomes. Multidisciplinary care, including oncologists, endocrinologists, and supportive care specialists, ensures comprehensive management of both cancer and treatment-related effects.
Emotional and Psychological Support
As patients transition out of immunotherapy, emotional and psychological well-being becomes a critical part of recovery. The end of active treatment often brings complex feelings—relief, uncertainty, fear of recurrence, or anxiety about the future. Many patients report feeling isolated or vulnerable, especially when regular contact with their care team decreases.
Providing structured emotional support is essential. Counseling services, both individual and group-based, can help patients process their experiences and navigate survivorship. Peer support groups, whether in-person or online, offer shared understanding and reduce feelings of loneliness. Additionally, access to mental health professionals trained in oncology—such as psycho-oncologists or social workers—can address depression, anxiety, or trauma related to cancer and its treatment.
Programs through hospitals, cancer centers, and national organizations like the American Cancer Society, CancerCare, and Livestrong offer tailored mental health resources. Encouraging patients to engage with these services not only improves quality of life but also supports their continued physical and emotional healing beyond treatment.
What Should Patients Discuss with Their Doctors?
After completing immunotherapy, it’s important for patients to stay informed and engaged in their follow-up care. Open communication with your healthcare team helps ensure a smooth recovery and early detection of any late effects. Here’s a brief checklist of important discussion points:
-
What is my follow-up schedule (e.g., imaging, blood tests)?
-
What long-term side effects should I watch for?
-
How will you monitor for cancer recurrence?
-
Do I need to continue any medications or hormone replacements?
-
Are there any lifestyle changes (diet, activity) I should follow?
-
When should I contact you about new symptoms?
-
Can I receive vaccinations or travel safely?
-
Are there any mental health or support resources you recommend?
-
Should I consider genetic counseling or further molecular testing?
-
When is it safe to resume work, exercise, or sexual activity?
Bringing this checklist to appointments can help guide conversations and ensure you don’t miss important aspects of your post-treatment care.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What happens after immunotherapy ends?
After immunotherapy, patients enter a monitoring phase to assess for cancer recurrence and manage any long-term side effects. Follow-up care often includes imaging, lab tests, and symptom tracking.
How long do side effects of immunotherapy last?
Some side effects, like fatigue or skin changes, may resolve within weeks, while others—such as thyroid dysfunction or joint pain—can persist long-term or even become permanent.
Can the thyroid recover after immunotherapy?
In many cases, thyroid function does not fully recover after immune-related thyroiditis. Most patients with hypothyroidism will need lifelong thyroid hormone replacement.
Is it normal to feel tired after immunotherapy?
Yes, fatigue is one of the most common side effects and can last for weeks or months. Proper hydration, rest, and gentle exercise can help manage symptoms.
What is the life expectancy after completing immunotherapy?
Life expectancy varies by cancer type and patient response. Some patients achieve durable remission lasting years, with clinical trials showing survival beyond 5–10 years in certain cancers.
Are long-term side effects common?
Yes, long-term effects like hormone imbalances, skin conditions, and joint pain can occur. Regular check-ups help detect and manage these issues early.
What tests are needed after immunotherapy ends?
Patients typically undergo imaging (CT, MRI, PET), blood tests (TSH, liver/kidney panels), and physical exams every 3–6 months, depending on their cancer and treatment response.
Can immunotherapy side effects appear after treatment stops?
Yes, immune-related side effects can develop weeks or even months after finishing therapy, which is why ongoing monitoring is essential.
Does experiencing side effects mean the treatment worked?
Some studies suggest that immune-related side effects may correlate with better treatment response, though this isn’t always the case and varies by individual.
How can patients support their recovery after immunotherapy?
Recovery is supported through balanced nutrition, regular physical activity, stress management, mental health support, and adherence to follow-up appointments.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
