
Zolbetuximab: Uses in Cancer, Side Effects, Dosages, Expectations, and More
Zolbetuximab is a monoclonal antibody that targets Claudin 18.2 (CLDN18.2), a tight-junction protein that is overexpressed in certain cancers, particularly gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinomas. On October 18, 2024, the U.S. Food and Drug Administration (FDA) approved Zolbetuximab for the treatment of locally advanced unresectable or metastatic HER2-negative and gastric gastroesophageal junction (GEJ) adenocarcinoma in patients whose tumors express Claudin 18.2 (CLDN18.2). It was approved for use in combination with fluoropyrimidine- and platinum-containing chemotherapy as a first-line treatment.
Which company produced Zolbetuximab?
Zolbetuximab (brand name Vyloy) was developed by Astellas Pharma Inc., a Japanese multinational pharmaceutical company. The company was established on April 1, 2005, following the merger of Yamanouchi Pharmaceutical Co., Ltd., and Fujisawa Pharmaceutical Co., Ltd. Astellas focuses on therapeutic areas such as oncology, urology, immunology, ophthalmology, and women’s health. The company is headquartered in Tokyo, Japan, and is known for developing innovative medicines for unmet medical needs.
How does Zolbetuximab work?
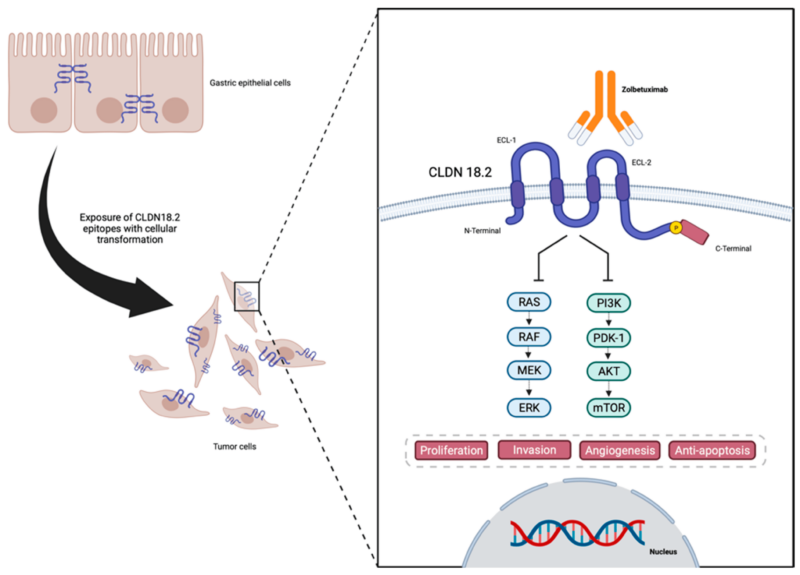
Zolbetuximab is a monoclonal antibody designed to target Claudin 18.2 (CLDN18.2), a protein normally found in the tight junctions of gastric mucosal cells. Claudin 18.2 (CLDN18.2) is a membrane protein found in the lining of the stomach, where it helps maintain the tight junctions between cells. These junctions act like biological glue, keeping cells tightly packed together to form a protective barrier. Under normal conditions, Claudin 18.2 remains hidden within these junctions, shielded from the immune system.
Cecília, M., De Jesus, V. H., Jácome, A., Donadio, M. D., Aruquipa, M. P., Fogacci, J., Cunha, R. G., Da Silva, L. M., & Peixoto, R. D. (2023). Claudin 18.2 as a New Biomarker in Gastric Cancer—What Should We Know? Cancers, 16(3), 679. https://doi.org/10.3390/cancers16030679
However, in certain cancers, such as gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinomas, changes in the structure of tumor cells cause Claudin 18.2 to become abnormally exposed on the cell surface. This exposure occurs because cancer disrupts the normal integrity of tight junctions, allowing Claudin 18.2 to be detected outside the cell.
This unique characteristic makes it an ideal therapeutic target for Zolbetuximab. Once administered, zolbetuximab selectively binds to Claudin 18.2 on tumor cells, marking them for destruction. This triggers the body’s immune system to attack the cancer cells through two key mechanisms:
- Antibody-Dependent Cellular Cytotoxicity (ADCC):
After attaching to the tumor cells, zolbetuximab recruits and activates immune effector cells, such as natural killer (NK) cells and macrophages. These immune cells then attack and destroy the tumor cells, leading to a reduction in tumor burden. - Complement-Dependent Cytotoxicity (CDC):
Zolbetuximab also activates the complement system, a part of the immune response that enhances the ability to clear pathogens and damaged cells. This activation results in the formation of the membrane attack complex (MAC), which punctures the tumor cell membrane, ultimately leading to cell lysis and death.
By leveraging these immune-mediated processes, zolbetuximab helps the body recognize and eliminate cancer cells more effectively. Its selectivity for Claudin 18.2-positive tumors ensures that healthy tissues remain largely unaffected, minimizing unwanted side effects.
What Cancers is Zolbeduximab Approved to Treat?
Zolbetuximab is an innovative treatment designed for patients with advanced gastric (stomach) and gastroesophageal junction (GEJ) adenocarcinoma. The treatment is specifically for HER2-negative tumors that express CLDN18.2, a protein found in certain cancer cells. These cancers can be particularly aggressive and difficult to treat, but zolbetuximab offers a targeted approach that helps the immune system recognize and attack tumor cells. Currently, the FDA has approved zolbetuximab as a first-line treatment for patients with locally advanced, unresectable, or metastatic gastric and GEJ adenocarcinoma. Learn more about stomach cancer, symptoms, and causes on OncoDaily.

Learn more about stomach cancer, symptoms, and causes on OncoDaily.
What research is behind the approval?
This approval was primarily based on the results of two pivotal Phase 3 clinical trials:
- SPOTLIGHT Trial: A randomized, double-blind, multicenter study evaluating zolbetuximab in combination with mFOLFOX6 chemotherapy versus placebo with mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma. The trial met its primary endpoint, demonstrating a significant improvement in progression-free survival (PFS) for patients receiving zolbetuximab.
- GLOW Trial: This randomized, double-blind, multicenter study assessed zolbetuximab combined with CAPOX chemotherapy compared to a placebo with CAPOX in a similar patient population. The GLOW trial also achieved its primary endpoint, showing a notable enhancement in PFS for the zolbetuximab group.
Both studies highlighted the efficacy of zolbetuximab in prolonging PFS when used alongside standard chemotherapy regimens in patients with CLDN18.2-positive gastric or GEJ adenocarcinoma.
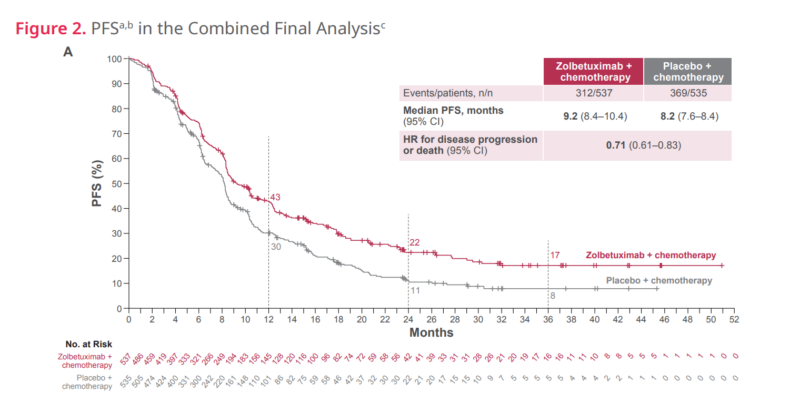
The final pooled analysis of the SPOTLIGHT (NCT03504397) and GLOW (NCT03653507) trials was presented as Poster 1438P at the ESMO Congress 2024 on September 14, 2024, during Poster Session 18 (Gastric & Gastro-Oesophageal Junction Cancer). Patients receiving zolbetuximab achieved a median progression-free survival (PFS) of 9.2 months (vs. 8.2 months with placebo, HR 0.71, P < 0.001) and a median overall survival (OS) of 16.4 months (vs. 13.7 months with placebo, HR 0.77, P < 0.001). The results supported FDA approval of zolbetuximab as a first-line standard of care for CLDN18.2-positive gastric and GEJ adenocarcinoma.
In conjunction with zolbetuximab’s approval, the FDA also approved the VENTANA CLDN18 (43-14A) RxDx Assay as a companion diagnostic device. This assay is designed to identify patients whose tumors express CLDN18.2, ensuring appropriate selection for zolbetuximab therapy.
Zolbetuximab Combinations and Treatment Outcomes
Zolbetuximab (Vyloy™) is used in combination with standard chemotherapy for the treatment of CLDN18.2-positive, HER2-negative gastric and gastroesophageal junction (GEJ) adenocarcinoma. Chemotherapy regimen studies, such as the SPOTLIGHT trial (mFOLFOX6+Zolbetuximab) and GLOW trial(CAPOX+Zolbetuximab), showed a significant PFS improvement. Besides the improvement of PFS, it is important to discuss adverse events. In the SPOTLIGHT trial, the most frequently reported serious adverse effects (≥2% incidence) included vomiting, nausea, neutropenia, febrile neutropenia, diarrhea, intestinal obstruction, fever (pyrexia), pneumonia, respiratory failure, pulmonary embolism, loss of appetite, and sepsis. In the GLOW trial, common serious adverse reactions (≥2% incidence) were vomiting, nausea, reduced appetite, low platelet count, upper gastrointestinal bleeding, diarrhea, pneumonia, pulmonary embolism, and fever (pyrexia).
Learn more about Esophageal cancer, symptoms, and causes on OncoDaily.
The Side Effects of Zolbetuximab and its Management
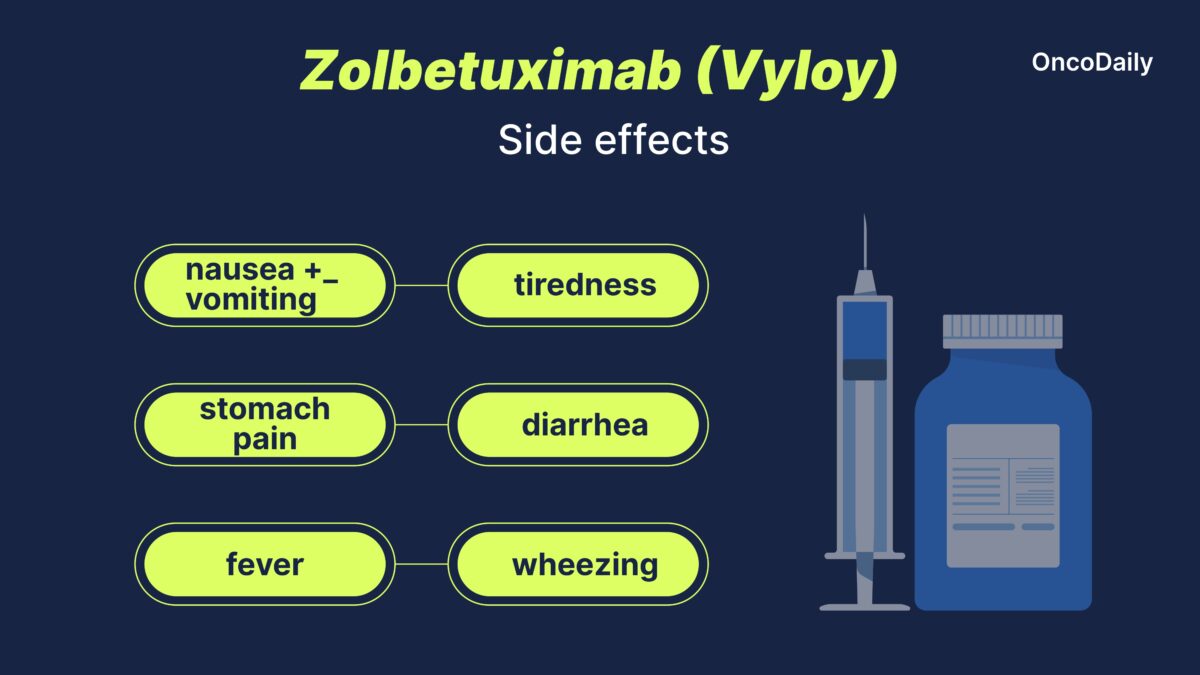
Zolbetuximab can cause gastrointestinal issues, fatigue, blood abnormalities, and infusion-related reactions, requiring careful monitoring and supportive care.
- Gastrointestinal Issues: includes nausea, vomiting, diarrhea, constipation, abdominal pain, loss of appetite, and weight loss. Managed with anti-nausea medications, hydration, and dietary adjustments.
- Fatigue & Weakness: Causes low energy and muscle weakness.
- Peripheral Neuropathy: Symptoms include tingling, numbness, and burning in hands and feet. Managed with dose adjustments, vitamins, and physical therapy.
- Fever (Pyrexia): Can indicate infection risk. Treated with fever-reducing medications and hydration.
- Blood Cell Count Reductions: Increases infection and bleeding risk. Requires regular monitoring, growth factor support, or transfusions.
- Electrolyte Imbalances: These can cause muscle cramps, weakness, or irregular heartbeat. Managed with electrolyte replacement and IV fluids.
- Severe Nausea & Vomiting: This may lead to dehydration and nutrition issues. Controlled with anti-nausea medications and IV hydration.
- Infusion-Related Reactions: Rare but serious, including allergic responses like rash or difficulty breathing. This can be prevented with pre-medication and close monitoring.
To improve tolerability, oncologists may adjust treatment by modifying infusion rates, temporarily pausing therapy, or reducing the dose based on the severity of side effects. Additionally, early intervention with supportive care—such as anti-nausea medications, blood transfusions, and electrolyte management—can help patients stay on treatment while maintaining their quality of life.
What is the Recommended Dosage of Zobetuximab?
Zolbetuximab (Vyloy™) is administered intravenously, with its dosage carefully determined based on the patient’s body surface area (BSA). The treatment begins with an initial dose of 800 mg/m², given as an intravenous infusion. Following this, maintenance doses are adjusted to 600 mg/m² every three weeks or 400 mg/m² every two weeks, depending on the specific treatment protocol.
How is Zobetuximab administered?
Zolbetuximab is administered intravenously. Before each infusion, antiemetics should be given to prevent nausea and vomiting. Zolbetuximab should be infused through a 0.2-micrometer in-line filter and must not be given as a bolus or mixed with other IV drugs. If administered alongside fluoropyrimidine- and platinum-based chemotherapy, zolbetuximab should be given first. Patients require close monitoring for hypersensitivity or infusion reactions. If severe, the infusion may be paused or discontinued based on severity.
Zolbetuximab vials should be refrigerated at 2°C to 8°C (36°F to 46°F) in their original carton and must not be frozen or shaken. Once reconstituted, the vials can be kept at room temperature (15°C to 30°C / 59°F to 86°F) for up to 5 hours if not used immediately. For prepared infusion bags, storage depends on temperature. If kept at room temperature, they should be used within 6 hours from preparation to infusion completion. If refrigerated (2°C to 8°C / 36°F to 46°F), they can be stored for up to 16 hours. Freezing must be avoided at all times to maintain stability.
What to Avoid During Zobetuximab Treatment?
Patients receiving zolbetuximab should take precautions to reduce risks and ensure treatment effectiveness. Live vaccines should be avoided as the drug may weaken the immune system, and immunosuppressive medications could interfere with its effects. Alcohol may worsen nausea and liver issues, while raw or undercooked foods increase infection risks. Pregnant women should not receive zolbetuximab, and effective contraception is recommended during and after treatment. Breastfeeding is also discouraged due to potential risks. To prevent infections, patients should practice good hygiene, avoid sick individuals, and limit exposure to crowded places. Lastly, skipping or stopping treatment without consulting a doctor may reduce its effectiveness, so any concerns should be discussed with a healthcare provider.
How effective is Vyloy (Zobetuximab)?
Zolbetuximab has shown significant efficacy in CLDN18.2-positive, HER2-negative gastric and gastroesophageal junction (GEJ) adenocarcinomas. The SPOTLIGHT and GLOW Phase III trials demonstrated that zolbetuximab combined with mFOLFOX6 or CAPOX chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone【ESMO】. Based on these results, the FDA approved zolbetuximab (Vyloy) on October 18, 2024, for first-line treatment in this patient group.
Ongoing trials with Zobetuximab
Following Zolbetuximab’s success in advanced gastric and GEJ adenocarcinomas, ongoing studies are now exploring its use in neoadjuvant settings, combination therapies, and other cancer types. Key trials include NEO-CLAUD (NCT06732856) for locally advanced gastric cancer, NCT06396091 for metastatic pancreatic cancer, and additional studies evaluating its safety, efficacy, and optimal dosing strategies. The NCT06396091 trial is testing zolbetuximab + chemotherapy in adults with metastatic pancreatic cancer whose tumors express Claudin 18.2 (CLDN18.2). This global study aims to determine the optimal dose, safety, and survival benefits of zolbetuximab. The trial has two phases: an initial Safety Lead-in Phase, where patients receive different zolbetuximab doses with chemotherapy, followed by a Randomization Phase, where participants receive either zolbetuximab + chemotherapy or chemotherapy alone. Treatment is given via IV infusion every 4 weeks, with regular health monitoring, blood tests, and imaging. Eligible patients must have untreated metastatic pancreatic cancer with CLDN18.2 expression, while those with recent radiotherapy, immune suppression, or brain metastases are excluded.
The NEO-CLAUD trial is evaluating zolbetuximab combined with docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy as a neoadjuvant treatment for locally advanced gastric cancer (LAGC). Since Claudin 18.2 is overexpressed in nearly half of operable gastric cancers, targeting it with zolbetuximab may enhance treatment effectiveness. This study aims to assess its safety and potential to reduce recurrence and improve survival in patients with Claudin 18.2-positive LAGC. These trials aim to expand zolbetuximab’s role in cancer treatment and improve patient outcomes.
Written by Mariam Khachatryan, MD
FAQ
What is Zolbetuximab?
Zolbetuximab is a monoclonal antibody that targets claudin-18.2, a protein found in certain gastrointestinal cancers. It is designed to help the immune system attack and destroy cancer cells expressing this protein.
Which company developed Zolbetuximab?
Zolbetuximab was developed by Astellas Pharma, a Japanese pharmaceutical company specializing in oncology and innovative drug therapies.
How does Zolbetuximab differ from other gastric cancer treatments?
Unlike chemotherapy, which targets rapidly dividing cells, Zolbetuximab specifically binds to claudin-18.2 on cancer cells, making it a more targeted therapy with potentially fewer off-target effects.
Is Zolbetuximab used alone or in combination with other therapies?
Zolbetuximab is typically used in combination with chemotherapy, such as FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) and CAPOX to enhance treatment efficacy in patients with claudin-18.2-positive gastric and gastroesophageal junction cancers.
What are the most common side effects of Zolbetuximab?
The most common side effects include nausea, vomiting, fatigue, decreased appetite, and low blood cell counts. More severe side effects may include infusion reactions and gastrointestinal disturbances.
How is Zolbetuximab administered?
Zolbetuximab is given as an intravenous (IV) infusion in a clinical setting. The dosing schedule depends on the treatment regimen and the patient's overall health condition.
Are there ongoing clinical trials for Zolbetuximab?
Yes, ongoing trials are exploring its use in combination with other treatments and its effectiveness in additional cancers, such as pancreatic and other gastrointestinal malignancies.
Can Zolbetuximab be used for cancers other than gastric and GEJ adenocarcinoma?
Currently, Zolbetuximab is only FDA-approved for HER2-negative, CLDN18.2-positive gastric and GEJ adenocarcinomas. However, clinical trials are exploring its potential in pancreatic cancer and other CLDN18.2-expressing tumors.
How is a patient determined to be eligible for Zolbetuximab treatment?
Before starting Zolbetuximab, patients must undergo a diagnostic test to confirm CLDN18.2 expression in their tumor. The FDA-approved VENTANA CLDN18 (43-14A) RxDx Assay is used to determine eligibility.
Are there any drug interactions to be aware of while on Zolbetuximab?
Patients should avoid live vaccines and immunosuppressive medications, as these may interfere with the immune response. Alcohol consumption should be limited due to potential gastrointestinal side effects.
What is the cost of Zolbetuximab, and is it covered by insurance?
The cost varies based on location, dosage, and healthcare system. Many insurance plans may cover Zolbetuximab for FDA-approved indications, but prior authorization may be required. Financial assistance programs from Astellas Pharma may also be available.
How long does Zolbetuximab treatment typically last?
Treatment duration depends on individual response and tolerance. Patients receive Zolbetuximab every two or three weeks via intravenous infusion, continuing as long as there is clinical benefit and manageable side effects.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
