
Elvina Almuradova: Dual-HER2 inhibition with trastuzumab + pertuzumab in HER2-positive mCRC
Elvina Almuradova, Associate Professor of Oncology at IEO (European Institute of Oncology) shared on LinkedIn:
“Dual-HER2 inhibition with trastuzumab + pertuzumab shows promise as a safe, chemo-free option in HER2-positive mCRC, with greater benefit in higher HER2 amplification vs. standard anti-EGFR therapy:
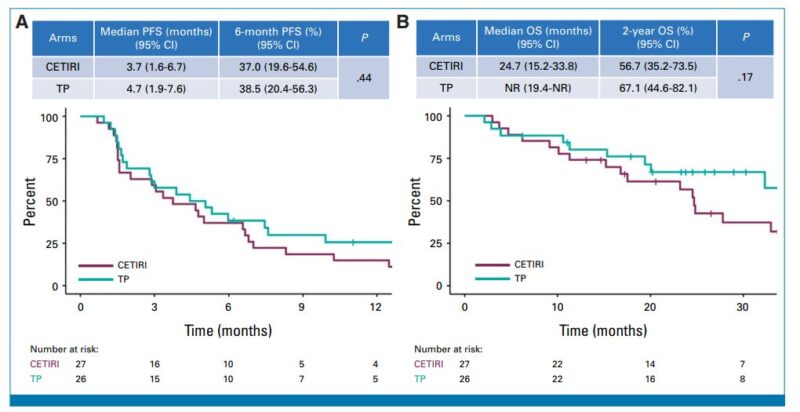
mPFS :
TP: 4.7 months (95% CI: 1.9–7.6).
CETIRI: 3.7 months (95% CI: 1.6–6.7).
HER2 Gene Copy Number (GCN):
Significant impact on efficacy.
GCN ≥20: TP outperformed CETIRI (PFS: 9.9 vs. 2.9 months).
GCN <20: CETIRI slightly better than TP (PFS: 4.2 vs. 3.0 months).
ORR:
Higher efficacy in GCN ≥20 (ORR: 57.1%) vs. GCN <20 (ORR: 9.1%).
Adverse Events (Grade ≥3):
TP: 23.1%.
CETIRI: 46.1%.
NOT: The study highlights precision in treatment selection is critical for HER2-positive mCRC. While dual-HER2 inhibition with trastuzumab and pertuzumab appears to be a safe and effective alternative to standard anti-EGFR therapy, HER2 amplification levels significantly influence treatment outcomes.”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
