
Behnam Nabet: Establishing a versatile approach for creating transgenic dTAG mice to degrade oncoproteins
Behnam Nabet, Assistant Professor at Fred Hutchinson Cancer Center, shared a recent article on X:
“Ready, set, degrade.
So proud to share our work in Journal of Clinical Investigation, establishing a versatile approach for creating transgenic dTAG mice to degrade oncoproteins.
We showcase that KRAS G12V degradation triggers antitumor immunity in lung cancer.”
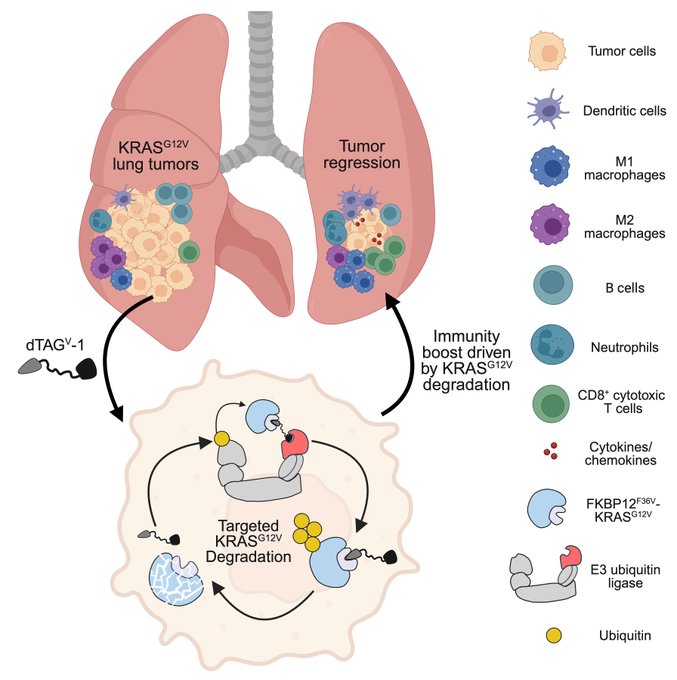
“This work was a tremendous collaboration between my lab, Hua Zhang, Kwok Wong’s lab, and led by Dezhi, Ke, Yuan, Jiajia, and Saurav Kumar.
Special shoutout to Nabet lab members, including Annabel Olson, Christina, Joy and Mia for their hard work.”

“Our goal was to degrade KRAS G12V, a driver of lung and pancreatic cancers that has not been selectively drugged.
To evaluate the consequences of destroying KRAS G12V in vivo, we aimed to establish a facile approach for degrading oncoproteins in transgenic mice.”
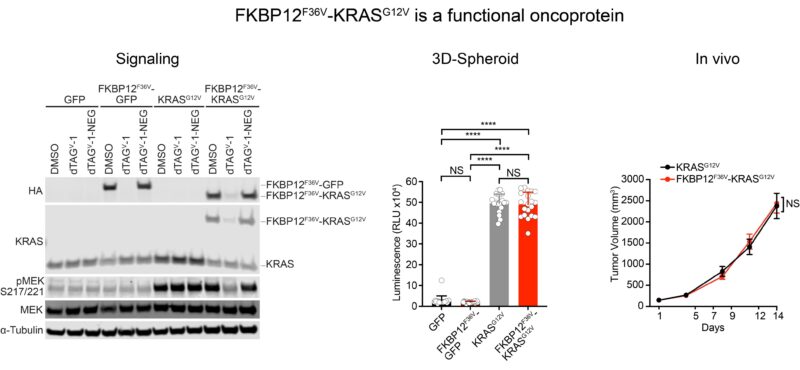
“Over the years, we developed several dTAG cellular models to degrade mutant KRAS.
Here, we first confirmed that tagging KRAS G12V did not alter protein function. dTAG-KRAS G12V activated signaling, transformed cells, and drove tumor formation, comparable to untagged KRAS G12V.”
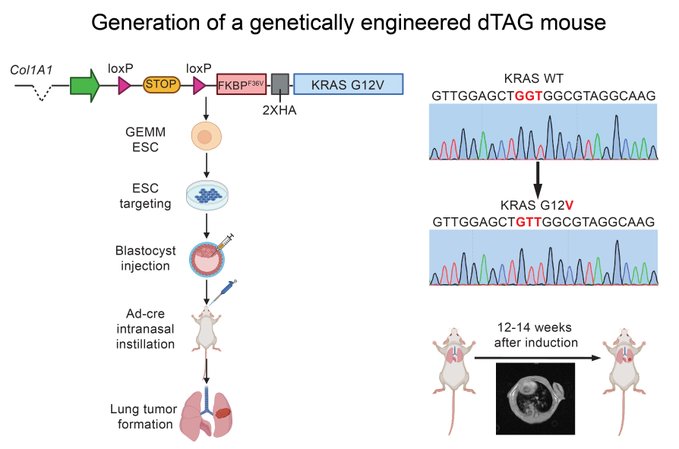
“To develop a transgenic dTAG-KRAS G12V mouse model, we designed a targeting vector that included a Lox-Stop-Lox cassette to allow for spatial/temporal control of dTAG-KRAS G12V. We activated dTAG-KRAS G12V with a Cre recombinase in the lung, resulting in lung adenocarcinoma.”
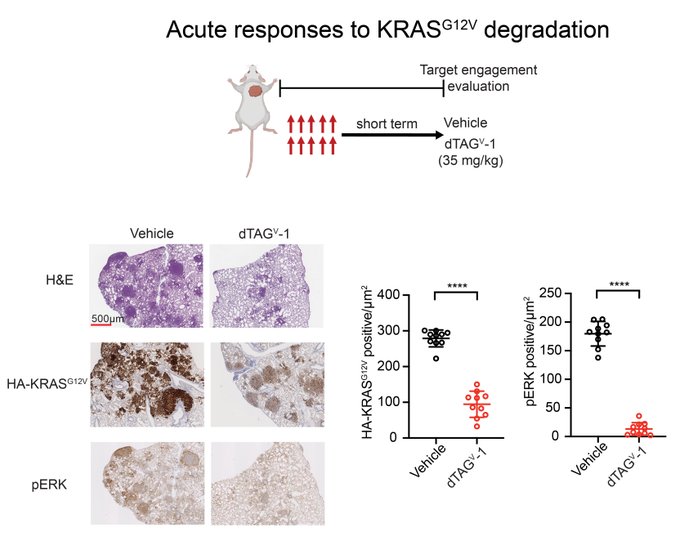
“Gratifyingly, acute administration of dTAG molecules led to effective KRAS G12V degradation in the tumor, collapsing downstream signaling, decreasing proliferation and increasing cell death.”
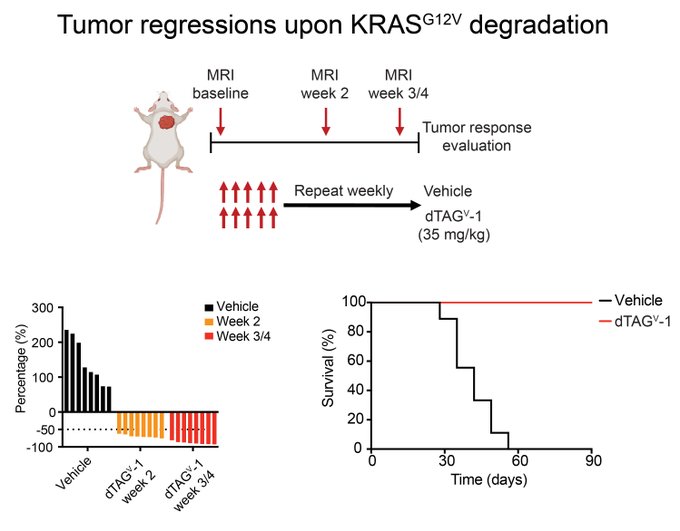
“To evaluate the consequences of prolonged KRAS G12V degradation, we next performed efficacy studies upon dTAG molecule administration.
We were thrilled to see remarkable tumor regressions upon KRAS G12V degradation.”
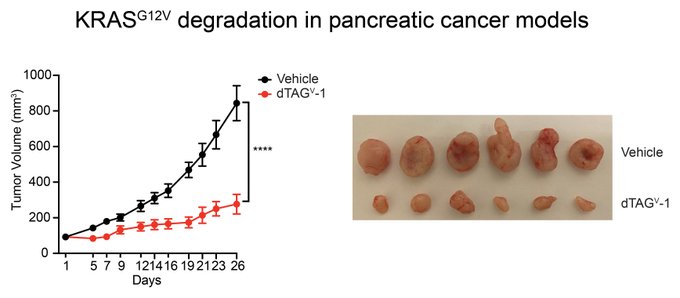
“To extend our observations to pancreatic cancer, we turned to an isogenic pancreatic cancer xenograft model.
Excitingly, we found that targeted degradation of KRAS G12V effectively decreased tumor burden in this pancreatic cancer model.”
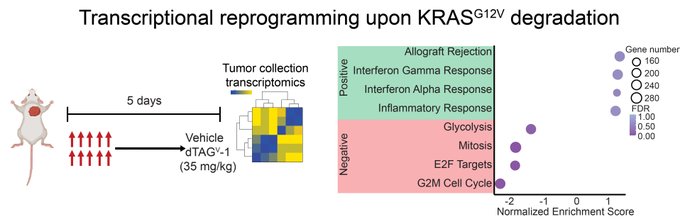
” Our immune-competent model allows us to determine the tumor intrinsic and extrinsic mechanisms leading to tumor regression.
Using bulk RNA-sequencing, we observed that acute KRAS G12V degradation led to upregulation of gene signatures associated with the inflammatory response.”
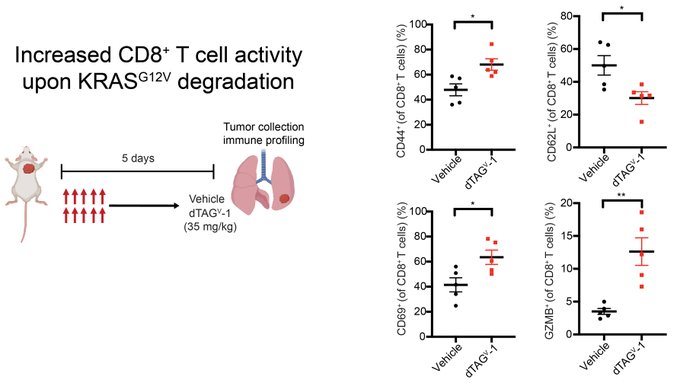
“This result inspired us to use immune profiling to dig deeper.
We found that KRAS G12V degradation increased overall immune cell infiltration and increased CD8+ T activity.”
“Turning to single-cell RNA-sequence, we found that KRAS G12V degradation reprogramed the tumor microenvironment to promote antitumor immunity.”
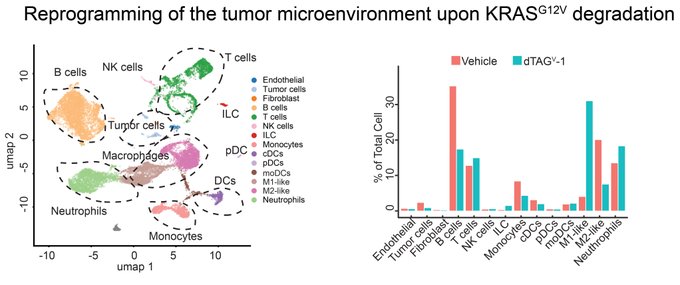
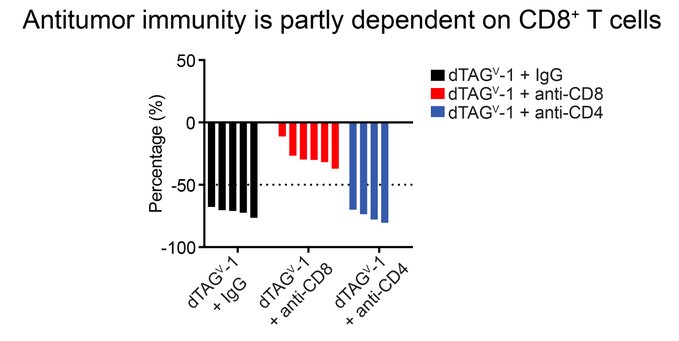
” Finally, our profiling studies highlighted the key role of T cells in these responses and we evaluated the direct effects of CD8+ or CD4+ T cells.
Neutralization of CD8+ T cells largely rescued these responses, highlighting the role of CD8+ T cells in antitumor immunity.”
“We hope that our study inspires applications of the dTAG system for modeling the pharmacological consequences of targeted oncoprotein degradation in transgenic mice.
We remain very excited about the applications of degraders for targeting KRAS mutant cancers.”
“Also, check out a new preprint from Cris Mayor-Ruiz and Santamaria’s Lab applying the dTAG system to develop syngeneic KRAS G12V lung cancer models.”
“Many thanks to our highly collaborative team and all co-authors.
Fred Hutchinson Cancer Center, University of Pittsburgh, New York University, Sarafan ChEM-H and Stanford Medicine.
We also thank our funding sources including National Cancer Institute and NIH.
Happy holidays to all.”
Title: Targeted degradation of oncogenic KRASG12V triggers antitumor immunity in lung cancer models
Authors: Dezhi Li, Ke Geng, Yuan Hao, Jiajia Gu, Saurav Kumar, Annabel T. Olson, Christina C. Kuismi, Hye Mi Kim, Yuanwang Pan, Fiona Sherman, Asia M. Williams, Yiting Li, Fei Li, Ting Chen, Cassandra Thakurdin, Michela Ranieri, Mary Meynardie, Daniel S. Levin, Janaye Stephens, Alison Chafitz, Joy Chen, Mia S. Donald-Paladino, Jaylen M. Powell, Ze-Yan Zhang, Wei Chen, Magdalena Ploszaj, Han Han, Shengqing Gu, Tinghu Zhang, Baoli Hu, Benjamin A. Nacev, Medard Ernest Kaiza, Alice H. Berger, Xuerui Wang, Jing Li, Xuejiao Sun, Yang Liu, Xiaoyang Zhang, Tullia C. Bruno, Nathanael S. Gray, Behnam Nabet, Kwok-Kin Wong and Hua Zhang

Behnam Nabet, Ph.D. is an Assistant Professor at Fred Hutchinson Cancer Research Center, where he leads the Nabet Lab focused on targeting oncogenic signaling networks through protein homeostasis to develop new cancer therapies. His team employs innovative degradation-based technologies to discover and validate clinically relevant targets, aiming to advance cancer treatments. Dr. Nabet is committed to collaborative science and mentoring the next generation of researchers.
Before joining Fred Hutch, Dr. Nabet was a Katherine Loker Pinard Fellow at Dana-Farber Cancer Institute, where he developed the dTAG system, a groundbreaking technology that enables rapid degradation of target proteins for drug discovery and target validation. His work, supported by prestigious fellowships from the American Cancer Society and the Claudia Adams Barr Program, has been published in leading journals like Nature, Cell, and Cancer Cell.
For more updates, follow OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
