Mafalda Oliveira MD, PhD is a highly respected medical oncologist at the Vall d’Hebron University Hospital and the Vall d’Hebron Institute of Oncology (VHIO) in Barcelona, where she has been practicing since 2011. She completed her Master in Clinical Research in June 2013 and her PhD in Medicine in July 2017, both at the Universidad Autònoma de Barcelona.
Dr. Oliveira’s research is focused on the molecular alterations and evolution of metastatic breast cancer, particularly on the clinical development of new drugs. She specializes in designing clinical trials that explore innovative biological hypotheses. Additionally, Dr. Oliveira is deeply involved in the application of liquid biopsies as diagnostic, predictive, and prognostic tools in breast cancer.
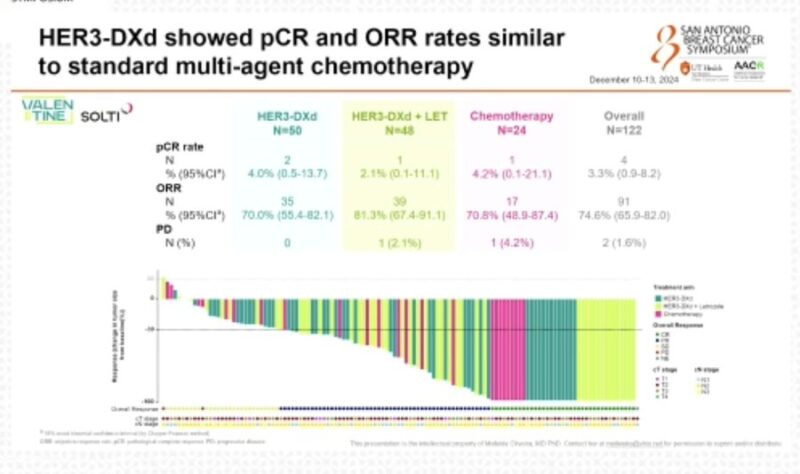
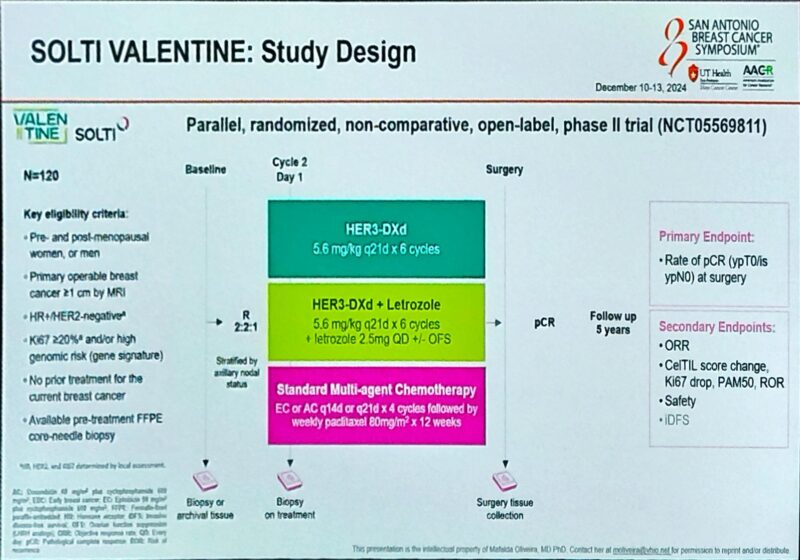
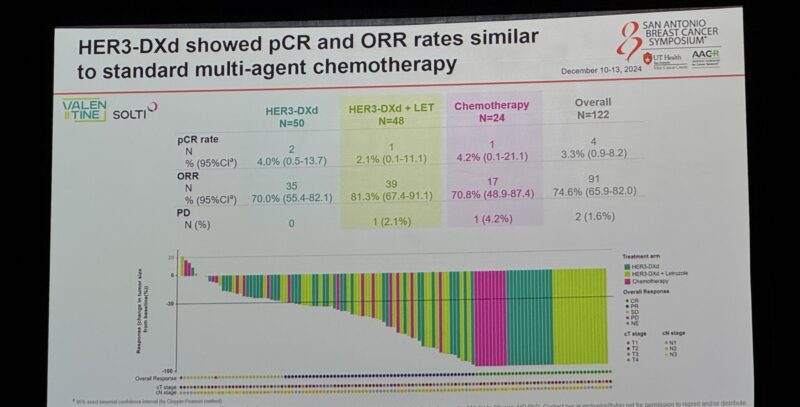
As Principal Investigator, she leads multiple clinical trials in breast cancer, spanning phase I, II, and III studies. These trials explore a variety of promising treatments, including drugs targeting the PI3K/AKT/mTOR pathway, CDK4/6 inhibitors, oral SERDs, new epigenetic therapies, antibody-drug conjugates (ADCs), and cancer immunotherapy.
Beyond her clinical and research roles, Dr. Oliveira is an active member of the Executive Board and Scientific Committee of the SOLTI-Breast Cancer Research Group, an academic cooperative research group in Spain. She is also involved with major professional organizations, including ASCO, ESMO, SEOM, and AACR.